Two-week administration of engineered Escherichia coli establishes persistent resistance to diet-induced obesity even without antibiotic pre-treatment
- PMID: 31203417
- PMCID: PMC7066869
- DOI: 10.1007/s00253-019-09958-x
Two-week administration of engineered Escherichia coli establishes persistent resistance to diet-induced obesity even without antibiotic pre-treatment
Abstract
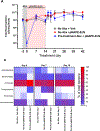
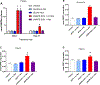
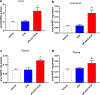
Adverse alterations in the composition of the gut microbiota have been implicated in the development of obesity and a variety of chronic diseases. Re-engineering the gut microbiota to produce beneficial metabolites is a potential strategy for treating these chronic diseases. N-acyl-phosphatidylethanolamines (NAPEs) are a family of bioactive lipids with known anti-obesity properties. Previous studies showed that administration of Escherichia coli Nissle 1917 (EcN) engineered with Arabidopsis thaliana NAPE synthase to produce NAPEs imparted resistance to obesity induced by a high-fat diet that persisted after ending their administration. In prior studies, mice were pre-treated with ampicillin prior to administering engineered EcN for 8 weeks in drinking water. If use of antibiotics and long-term administration are required for beneficial effects, implementation of this strategy in humans might be problematic. Studies were therefore undertaken to determine if less onerous protocols could still impart persistent resistance and sustained NAPE biosynthesis. Administration of engineered EcN for only 2 weeks without pre-treatment with antibiotics sufficed to establish persistent resistance. Sustained NAPE biosynthesis by EcN was required as antibiotic treatment after administration of the engineered EcN markedly attenuated its effects. Finally, heterologous expression of human phospholipase A/acyltransferase-2 (PLAAT2) in EcN provided similar resistance to obesity as heterologous expression of A. thaliana NAPE synthase, confirming that NAPEs are the bioactive mediator of this resistance.
Keywords: Antibiotics; Engineered bacteria; Gut microbiota; N-acyl-ethanolamides; N-acyl-phosphatidylethanolamines; N-acyltransferases.
Conflict of interest statement
Figures



References
-
- Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Dobele S, Tacconelli E (2017) Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 17(9):990–1001 doi:10.1016/S1473-3099(17)30325-0 - DOI - PubMed
-
- Chapman KD (2000) Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem Phys Lipids 108(1–2):221–229 - PubMed
-
- Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D, McGuinness OP, Niswender KD, Davies SS (2014) Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest 124(8):3391–33406 doi:10.1172/JCI72517 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- AT007830/AT/NCCIH NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- U2C DK059637/DK/NIDDK NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- RR030956/Foundation for the National Institutes of Health
- U24 DK059637/DK/NIDDK NIH HHS/United States
- DK059637/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 AT007830/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

