Acetylenes: cytochrome P450 oxidation and mechanism-based enzyme inactivation
- PMID: 31203694
- PMCID: PMC6699906
- DOI: 10.1080/03602532.2019.1632891
Acetylenes: cytochrome P450 oxidation and mechanism-based enzyme inactivation
Abstract
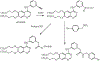
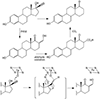
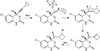
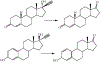
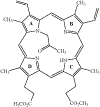
The oxidation of carbon-carbon triple bonds by cytochrome P450 produces ketene metabolites that are hydrolyzed to acetic acid derivatives or are trapped by nucleophiles. In the special case of 17α-ethynyl sterols, D-ring expansion and de-ethynylation have been observed as competing pathways. The oxidation of acetylenic groups is also associated with mechanism-based inactivation of cytochrome P450 enzymes. One mechanism for this inactivation is reaction of the ketene metabolite with cytochrome P450 residues essential for substrate binding or catalysis. However, in the case of monosubstituted acetylenes, inactivation can also occur by addition of the oxidized acetylenic function to a nitrogen of the heme prosthetic group. This addition reaction is not mediated by the ketene metabolite, but rather occurs during oxygen transfer to the triple bond. In some instances, a detectable intermediate is formed that is most consistent with a ketocarbene-iron heme complex. This complex can progress to the N-alkylated heme or revert back to the unmodified enzyme. The ketocarbene complex may intervene in the formation of all the N-alkyl heme adducts, but is normally too unstable to be detected.
Keywords: Acetylene oxidation; cytochrome P450 inactivation; ethynylsterols; iron–carbene complexes; ketene formation; oxirene.
Conflict of interest statement
Disclosure statement
No potential conflict of interest was reported by the author.
Figures



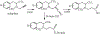
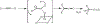

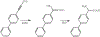

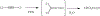


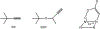
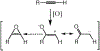

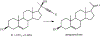
Similar articles
-
Aryl acetylenes as mechanism-based inhibitors of cytochrome P450-dependent monooxygenase enzymes.Chem Res Toxicol. 1997 Jan;10(1):91-102. doi: 10.1021/tx960064g. Chem Res Toxicol. 1997. PMID: 9074808
-
Determinants of protein modification versus heme alkylation: inactivation of cytochrome P450 1A1 by 1-ethynylpyrene and phenylacetylene.Chem Res Toxicol. 1993 Jan-Feb;6(1):38-45. doi: 10.1021/tx00031a006. Chem Res Toxicol. 1993. PMID: 8448348
-
Heme Modification Contributes to the Mechanism-Based Inactivation of Human Cytochrome P450 2J2 by Two Terminal Acetylenic Compounds.Drug Metab Dispos. 2017 Sep;45(9):990-999. doi: 10.1124/dmd.117.075846. Epub 2017 Jul 11. Drug Metab Dispos. 2017. PMID: 28698302 Free PMC article.
-
Mechanism-based inactivation and reversibility: is there a new trend in the inactivation of cytochrome p450 enzymes?Drug Metab Dispos. 2006 Jan;34(1):1-7. doi: 10.1124/dmd.105.004747. Drug Metab Dispos. 2006. PMID: 16369051 Review.
-
The oxidative inactivation of cytochrome P450 in monooxygenase reactions.Free Radic Biol Med. 1994 Jan;16(1):73-97. doi: 10.1016/0891-5849(94)90245-3. Free Radic Biol Med. 1994. PMID: 8299999 Review.
Cited by
-
The Functions of Cytochrome P450 ω-hydroxylases and the Associated Eicosanoids in Inflammation-Related Diseases.Front Pharmacol. 2021 Sep 14;12:716801. doi: 10.3389/fphar.2021.716801. eCollection 2021. Front Pharmacol. 2021. PMID: 34594219 Free PMC article. Review.
-
Time-dependent enzyme inactivation: Numerical analyses of in vitro data and prediction of drug-drug interactions.Pharmacol Ther. 2020 Feb;206:107449. doi: 10.1016/j.pharmthera.2019.107449. Epub 2019 Dec 11. Pharmacol Ther. 2020. PMID: 31836452 Free PMC article. Review.
-
Bioactivation and reactivity research advances - 2021 year in review.Drug Metab Rev. 2022 Aug;54(3):246-281. doi: 10.1080/03602532.2022.2097254. Epub 2022 Aug 5. Drug Metab Rev. 2022. PMID: 35876116 Free PMC article. Review.
-
Click biology highlights the opportunities from reliable biological reactions.Nat Chem Biol. 2025 Jul;21(7):991-1005. doi: 10.1038/s41589-025-01944-x. Epub 2025 Jun 19. Nat Chem Biol. 2025. PMID: 40537536 Review.
-
The Role of CYPs and Transporters in the Biotransformation and Transport of the Anti-hepatitis C Antiviral Agents Asunaprevir, Daclatasvir, and Beclabuvir: Impact of Liver Disease, Race and Drug-drug Interactions on Safety and Efficacy.Curr Drug Metab. 2024;25(2):96-109. doi: 10.2174/0113892002288832240213095622. Curr Drug Metab. 2024. PMID: 38441017 Review.
References
-
- Abdel-Aziz MT, Williams KIH. 1969. Metabolism of 17α-ethynylestradiol and its 3-methyl ether by the rabbit; an in vivo d-homoannulation. Steroids. 13:809–820. - PubMed
-
- Abdel-Aziz MT, Williams KIH. 1970. Metabolism of radioactive 17α-ethynylestradiol by women. Steroids 15:695–710. - PubMed
-
- Battioni P, Mahy JP, Delaforge M, Mansuy P. 1983. Reaction of monosubstituted hydrazines and diazenes with rat-liver cytochrome P450. Eur. J. Biochem 134:241–248. - PubMed
-
- Blakey DC, White INH. 1986. Destruction of cytochrome P-450 and formation of green pigments by contraceptive steroids in rat hepatocyte suspensions. Biochem Pharmacol. 35:1561–1567. - PubMed
-
- Blanksby SJ, Ellison GB. 2003. Bond dissociation energies of organic molecules. Accts Chem Res. 36:255–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
