Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation
- PMID: 31203721
- PMCID: PMC6999647
- DOI: 10.1080/15548627.2019.1628536
Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation
Abstract
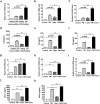
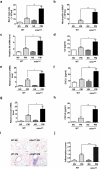
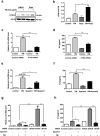
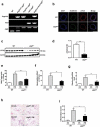
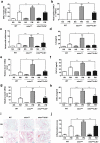
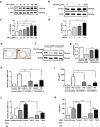
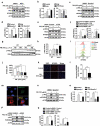
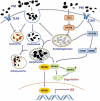
Particulate matter (PM) is able to induce airway epithelial injury, while the detailed mechanisms remain unclear. Here we demonstrated that PM exposure inactivated MTOR (mechanistic target of rapamycin kinase), enhanced macroautophagy/autophagy, and impaired lysosomal activity in HBE (human bronchial epithelial) cells and in mouse airway epithelium. Genetic or pharmaceutical inhibition of MTOR significantly enhanced, while inhibition of autophagy attenuated, PM-induced IL6 expression in HBE cells. Consistently, club-cell-specific deletion of Mtor aggravated, whereas loss of Atg5 in bronchial epithelium reduced, PM-induced airway inflammation. Interestingly, the augmented inflammatory responses caused by MTOR deficiency were markedly attenuated by blockage of downstream autophagy both in vitro and in vivo. Mechanistically, the dysregulation of MTOR-autophagy signaling was partially dependent on activation of upstream TSC2, and interacted with the TLR4-MYD88 to orchestrate the downstream NFKB activity and to regulate the production of inflammatory cytokines in airway epithelium. Moreover, inhibition of autophagy reduced the expression of EPS15 and the subsequent endocytosis of PM. Taken together, the present study provides a mechanistic explanation for how airway epithelium localized MTOR-autophagy axis regulates PM-induced airway injury, suggesting that activation of MTOR and/or suppression of autophagy in local airway might be effective therapeutic strategies for PM-related airway disorders.Abbreviations: ACTB: actin beta; AKT: AKT serine/threonine kinase; ALI: air liquid interface; AP2: adaptor related protein complex 2; ATG: autophagy related; BALF: bronchoalveolar lavage fluid; COPD: chronic obstructive pulmonary disease; CXCL: C-X-C motif chemokine ligand; DOX: doxycycline; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; EPS15: epidermal growth factor receptor pathway substrate 15; HBE: human bronchial epithelial; H&E: hematoxylin & eosin; IKK: IKB kinase; IL: interleukin; LAMP2: lysosomal-associated membrane protein 2; LPS: lipopolysaccharide; MAP1LC3B/LC3B: microtubule-associated protein 1 light chain 3 beta; MTEC: mouse tracheal epithelial cells; MTOR: mechanistic target of rapamycin kinase; MYD88: MYD88 innate immune signal transduction adaptor; NFKB: nuclear factor of kappa B; NFKBIA: NFKB inhibitor alpha; PM: particulate matter; PtdIns3K: phosphatidylinositol 3-kinase; Rapa: rapamycin; RELA: RELA proto-oncogene, NFKB subunit; SCGB1A1: secretoglobin family 1A member 1; siRNA: small interfering RNAs; SQSTM1: sequestosome 1; TEM: transmission electronic microscopy; TLR4: toll like receptor 4; TSC2: TSC complex subunit 2.
Keywords: Airway epithelial injury; MTOR; airway inflammation; autophagy; particulate matter.
Figures


References
-
- Guan WJ, Zheng XY, Chung KF, et al. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. - PubMed
-
- Santibanez-Andrade M, Quezada-Maldonado EM, Osornio-Vargas A, et al. Air pollution and genomic instability: the role of particulate matter in lung carcinogenesis. Environ Pollut. 2017;229:412–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
