A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory
- PMID: 31204082
- PMCID: PMC6687558
- DOI: 10.1016/j.neuron.2019.05.029
A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory
Abstract
Despite a wealth of clinical and preclinical data implicating the serotonin (5-HT) system in fear-related affective disorders, a precise definition of this neuromodulator's role in fear remains elusive. Using convergent anatomical and functional approaches, we interrogate the contribution to fear of basal amygdala (BA) 5-HT inputs from the dorsal raphe nucleus (DRN). We show the DRN→BA 5-HT pathway is engaged during fear memory formation and retrieval, and activity of these projections facilitates fear and impairs extinction. The DRN→BA 5-HT pathway amplifies fear-associated BA neuronal firing and theta power and phase-locking. Although fear recruits 5-HT and VGluT3 co-expressing DRN neurons, the fear-potentiating influence of the DRN→BA 5-HT pathway requires signaling at BA 5-HT1A/2A receptors. Input-output mapping illustrates how the DRN→BA 5-HT pathway is anatomically distinct and connected with other brain regions that mediate fear. These findings reveal how a discrete 5-HT circuit orchestrates a broader neural network to calibrate aversive memory.
Keywords: 5-HT; basal amygdala; dorsal raphe nucleus; fear conditioning; fear extinction; nucleus accumbens; serotonin.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
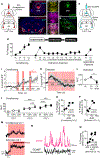
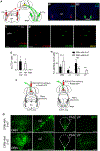
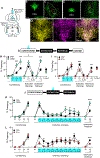
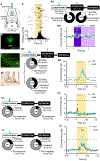



Comment in
-
The Ministry of Fear: 'The Conjuring' of Fright in the Amygdala by the Raphe.Neuron. 2019 Aug 7;103(3):356-358. doi: 10.1016/j.neuron.2019.07.020. Neuron. 2019. PMID: 31394058
References
-
- Akirav I, Raizel H, and Maroun M (2006). Enhancement of conditioned fear extinction by infusion of the GABA A agonist muscimol into the rat prefrontal cortex and amygdala. Eur. J. Neurosci 23, 758–764. - PubMed
-
- Allers KA, and Sharp T (2003). Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122, 193–204. - PubMed
-
- Ansorge MS, Zhou M, Lira A, Hen R, and Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science (80-. ) 306, 879–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

