Detection of Single-Nucleotide Polymorphism Markers of Antimalarial Drug Resistance Directly from Whole Blood
- PMID: 31204166
- PMCID: PMC6650786
- DOI: 10.1016/j.jmoldx.2019.02.004
Detection of Single-Nucleotide Polymorphism Markers of Antimalarial Drug Resistance Directly from Whole Blood
Abstract
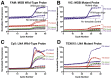
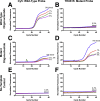
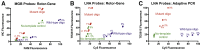
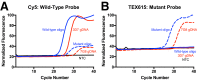
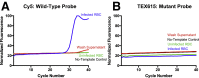
Monitoring of antimalarial resistance is important to prevent its further spread, but the available options for assessing resistance are less than ideal for field settings. Although molecular detection is perhaps the most efficient method, it is also the most complex because it requires DNA extraction and PCR instrumentation. To develop a more deployable approach, we designed new probes, which, when used in combination with an inhibitor-tolerant Taq polymerase, enable single-nucleotide polymorphism genotyping directly from whole blood. The probes feature two strategic design elements: locked nucleic acids to enhance specificity and the reporter dyes Cy5 and TEX615, which have less optical overlap with the blood absorbance spectra than other commonly used dyes. Probe performance was validated on a traditional laboratory-based instrument and then further tested on a field-deployable Adaptive PCR instrument to develop a point-of-care platform appropriate for use in malaria settings. The probes discriminated between wild-type Plasmodium falciparum and the chloroquine-resistant CRT PF3D7_0709000:c.227A>C (p.Lys76Thr) mutant in the presence of 2% blood. Additionally, in allelic discrimination plots with the new probes, samples clustered more closely to their respective axes compared with samples using minor groove binder probes with 6-FAM and VIC reporter dyes. Our strategy greatly simplifies single-nucleotide polymorphism detection and provides a more accessible alternative for antimalarial resistance surveillance in the field.
Copyright © 2019 American Society for Investigative Pathology and the Association for Molecular Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Wongsrichanalai C., Pickard A.L., Wernsdorfer W.H., Meshnick S.R. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. - PubMed
-
- Noedl H., Se Y., Schaecher K., Smith B.L., Socheat D., Fukuda M.M. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. - PubMed
-
- Dondorp A.M., Nosten F., Yi P., Das D., Phyo A.P., Tarning J., Lwin K.M., Ariey F., Hanpithakpong W., Lee S.J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S.S., Yeung S., Singhasivanon P., Day N.P., Lindegardh N., Socheat D., White N.J. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. - PMC - PubMed
-
- Phyo A.P., Nkhoma S., Stepniewska K., Ashley E.A., Nair S., McGready R., ler Moo C., Al-Saai S., Dondorp A.M., Lwin K.M., Singhasivanon P., Day N.P., White N.J., Anderson T.J., Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

