Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice
- PMID: 31204283
- PMCID: PMC6687560
- DOI: 10.1016/j.cmet.2019.05.015
Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice
Abstract
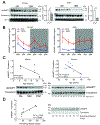
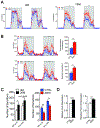
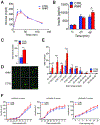
Aging is a significant risk factor for impaired tissue functions and chronic diseases. Age-associated decline in systemic NAD+ availability plays a critical role in regulating the aging process across many species. Here, we show that the circulating levels of extracellular nicotinamide phosphoribosyltransferase (eNAMPT) significantly decline with age in mice and humans. Increasing circulating eNAMPT levels in aged mice by adipose-tissue-specific overexpression of NAMPT increases NAD+ levels in multiple tissues, thereby enhancing their functions and extending healthspan in female mice. Interestingly, eNAMPT is carried in extracellular vesicles (EVs) through systemic circulation in mice and humans. EV-contained eNAMPT is internalized into cells and enhances NAD+ biosynthesis. Supplementing eNAMPT-containing EVs isolated from young mice significantly improves wheel-running activity and extends lifespan in aged mice. Our findings have revealed a novel EV-mediated delivery mechanism for eNAMPT, which promotes systemic NAD+ biosynthesis and counteracts aging, suggesting a potential avenue for anti-aging intervention in humans.
Keywords: EV; NAD+; adipose tissue; aging; eNAMPT; exosome; extracellular vesicle; hypothalamus; longevity; metabolism.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
S.I. receives a part of patent-licensing fees from MetroBiotech (USA) and Teijin Limited (Japan) through Washington University. R.S.A. is a co-founder of Metro Midwest Biotech. All other authors have no financial interests.
Figures




References
-
- Dahl TB, Holm S, Aukrust P, and Halvorsen B (2012). Visfatin/NAMPT: A Multifaceted Molecule with Diverse Roles in Physiology and Pathophysiology. Annu. Rev. Nutr. 32, 229–243. - PubMed
-
- Durcin M, Fleury A, Taillebois E, Hilairet G, Krupova Z, Henry C, Truchet S, Trotzmuller M, Kofeler H, Mabilleau G, et al. (2017). Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 6, 1305677. - PMC - PubMed
-
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, et al. (2007). Retraction. Science 318, 565b. - PubMed
-
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, and Kiess W (2015). Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 11, 535–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

