Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression
- PMID: 31204774
- PMCID: PMC7442325
- DOI: 10.1093/advances/nmz057
Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression
Abstract
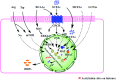
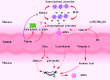
The intestinal tract is the shared locus of intestinal epithelial cells, immune cells, nutrient digestion and absorption, and microbial survival. The gut in animals faces continuous challenges in communicating with the external environment. Threats from endogenous imbalance and exogenous feeds, especially pathogens, could trigger a disorder of homeostasis, leading to intestinal disease and even systematic disease risk. As a part of the intestinal protective barrier, endogenous host defense peptides (HDPs) play multiple beneficial physiological roles in the gut mucosa. Moreover, enhancing endogenous HDPs is being developed as a new strategy for resisting pathogens and commensal microbes, and to maintain intestinal health and reduce antibiotic use. In recent years, multiple nutrients such as branched-chain amino acids, SCFAs, lactose, zinc, and cholecalciferol (vitamin D3) have been reported to significantly increase HDP expression. Nutritional intervention has received more attention and is viewed as a promising means to defend against pathogenic infections and intestinal inflammation. The present review focuses on current discoveries surrounding HDP expression and nutritional regulation of mechanisms in the gut. Our aim is to provide a comprehensive overview, referable tactics, and novel opinions.
Keywords: antibiotic alternative; gut; host defense peptides; inflammation; nutrients; pathogens.
Copyright © American Society for Nutrition 2019.
Figures

Similar articles
-
Does an Apple a Day Also Keep the Microbes Away? The Interplay Between Diet, Microbiota, and Host Defense Peptides at the Intestinal Mucosal Barrier.Front Immunol. 2020 Jun 9;11:1164. doi: 10.3389/fimmu.2020.01164. eCollection 2020. Front Immunol. 2020. PMID: 32655555 Free PMC article. Review.
-
CXCR2, as a key regulatory gene of HDP-PG-1, maintains intestinal mucosal homeostasis.Int J Biol Macromol. 2024 Jun;269(Pt 2):132025. doi: 10.1016/j.ijbiomac.2024.132025. Epub 2024 May 3. Int J Biol Macromol. 2024. PMID: 38704076
-
Sodium Butyrate Protects the Intestinal Barrier by Modulating Intestinal Host Defense Peptide Expression and Gut Microbiota after a Challenge with Deoxynivalenol in Weaned Piglets.J Agric Food Chem. 2020 Apr 15;68(15):4515-4527. doi: 10.1021/acs.jafc.0c00791. Epub 2020 Apr 2. J Agric Food Chem. 2020. PMID: 32208605
-
Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review.Adv Nutr. 2020 Jan 1;11(1):77-91. doi: 10.1093/advances/nmz061. Adv Nutr. 2020. PMID: 31268137 Free PMC article. Review.
-
Transcriptional Regulation of Antimicrobial Host Defense Peptides.Curr Protein Pept Sci. 2015;16(7):672-9. doi: 10.2174/1389203716666150630133432. Curr Protein Pept Sci. 2015. PMID: 26122785 Review.
Cited by
-
Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation.Antibiotics (Basel). 2023 Feb 9;12(2):361. doi: 10.3390/antibiotics12020361. Antibiotics (Basel). 2023. PMID: 36830272 Free PMC article. Review.
-
Immunometabolism, Micronutrients, and Bariatric Surgery: The Use of Transcriptomics and Microbiota-Targeted Therapies.Mediators Inflamm. 2020 Nov 17;2020:8862034. doi: 10.1155/2020/8862034. eCollection 2020. Mediators Inflamm. 2020. PMID: 33281501 Free PMC article. Review.
-
Butyrate, Forskolin, and Lactose Synergistically Enhance Disease Resistance by Inducing the Expression of the Genes Involved in Innate Host Defense and Barrier Function.Antibiotics (Basel). 2021 Sep 27;10(10):1175. doi: 10.3390/antibiotics10101175. Antibiotics (Basel). 2021. PMID: 34680756 Free PMC article.
-
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 3;9(1):6. doi: 10.1038/s41392-023-01679-y. Signal Transduct Target Ther. 2024. PMID: 38169461 Free PMC article. Review.
-
Epigenetic Regulation of Host Defense Peptide Synthesis: Synergy Between Histone Deacetylase Inhibitors and DNA/Histone Methyltransferase Inhibitors.Front Immunol. 2022 Apr 22;13:874706. doi: 10.3389/fimmu.2022.874706. eCollection 2022. Front Immunol. 2022. PMID: 35529861 Free PMC article.
References
-
- Varady J, Eder K, Ringseis R. Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. Eur J Nutr. 2011;50:601–9. - PubMed
-
- Pearce SC, Mani V, Weber TE, Rhoads RP, Patience JF, Baumgard LH, Gabler NK. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J Anim Sci. 2013;91:5183–93. - PubMed
-
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

