Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis
- PMID: 31204790
- PMCID: PMC6572872
- DOI: 10.1002/14651858.CD012334.pub2
Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis
Abstract
Background: Hepatic encephalopathy is a common complication of cirrhosis, with high related morbidity and mortality. Its presence is associated with a wide spectrum of change ranging from clinically obvious neuropsychiatric features, known as 'overt' hepatic encephalopathy, to abnormalities manifest only on psychometric or electrophysiological testing, 'minimal' hepatic encephalopathy. The exact pathogenesis of the syndrome is unknown but ammonia plays a key role. Drugs that specifically target ammonia include sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST-120 (spherical carbon adsorbent), and polyethylene glycol.
Objectives: To evaluate the beneficial and harmful effects of pharmacotherapies that specifically target ammonia versus placebo, no intervention, or other active interventions, for the prevention and treatment of hepatic encephalopathy in people with cirrhosis.
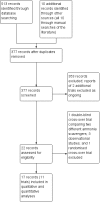
Search methods: We searched the Cochrane Hepato-Biliary Controlled Trials Register, CENTRAL, MEDLINE, Embase, and three other databases to March 2019. We also searched online trials registries such as ClinicalTrials.gov, European Medicines Agency, WHO International Clinical Trial Registry Platform, and the Food and Drug Administration for ongoing or unpublished trials. In addition, we searched conference proceedings, checked bibliographies, and corresponded with investigators.
Selection criteria: We included randomised clinical trials comparing sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST-120, and polyethylene glycol versus placebo or non-absorbable disaccharides, irrespective of blinding, language, or publication status. We included participants with minimal or overt hepatic encephalopathy or participants who were at risk of developing hepatic encephalopathy.
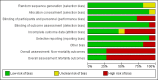
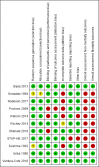
Data collection and analysis: Two review authors independently extracted data from the included reports. The primary outcomes were mortality, hepatic encephalopathy, and serious adverse events. We undertook meta-analyses and presented results using risk ratios (RR) or mean differences (MD), both with 95% confidence intervals (CIs), and I2 statistic values as a marker of heterogeneity. We assessed bias control using the Cochrane Hepato-Biliary domains and the certainty of the evidence using GRADE.
Main results: We identified 11 randomised clinical trials that fulfilled our inclusion criteria. Two trials evaluated the prevention of hepatic encephalopathy while nine evaluated the treatment of hepatic encephalopathy. The trials assessed sodium benzoate (three trials), glycerol phenylbutyrate (one trial), ornithine phenylacetate (two trials), AST-120 (two trials), and polyethylene glycol (three trials). Overall, 499 participants received these pharmacotherapies while 444 participants received a placebo preparation or a non-absorbable disaccharide. We classified eight of the 11 trials as at 'high risk of bias' and downgraded the certainty of the evidence to very low for all outcomes.Eleven trials, involving 943 participants, reported mortality data, although there were no events in five trials. Our analyses found no beneficial or harmful effects of sodium benzoate versus non-absorbable disaccharides (RR 1.26, 95% CI 0.49 to 3.28; 101 participants; 2 trials; I2 = 0%), glycerol phenylbutyrate versus placebo (RR 0.65, 95% CI 0.11 to 3.81; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.73, 95% CI 0.35 to 1.51; 269 participants; 2 trials; I2 = 0%), AST-120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial), or polyethylene glycol versus lactulose (RR 0.50, 95% CI 0.09 to 2.64; 190 participants; 3 trials; I2 = 0%).Seven trials involving 521 participants reported data on hepatic encephalopathy. Our analyses showed a beneficial effect of glycerol phenylbutyrate versus placebo (RR 0.57, 95% CI 0.36 to 0.90; 178 participants; 1 trial; number needed to treat for an additional beneficial outcome (NNTB) 6), and of polyethylene glycol versus lactulose (RR 0.19, 95% CI 0.08 to 0.44; 190 participants; 3 trials; NNTB 4). We did not observe beneficial effects in the remaining three trials with extractable data: sodium benzoate versus non-absorbable disaccharides (RR 1.22, 95% CI 0.51 to 2.93; 74 participants; 1 trial); ornithine phenylacetate versus placebo (RR 2.71, 95% CI 0.12 to 62.70; 38 participants; 1 trial); or AST-120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial).Ten trials, involving 790 participants, reported a total of 130 serious adverse events. Our analyses found no evidence of beneficial or harmful effects of sodium benzoate versus non-absorbable disaccharides (RR 1.08, 95% CI 0.44 to 2.68; 101 participants; 2 trials), glycerol phenylbutyrate versus placebo (RR 1.63, 95% CI 0.85 to 3.13; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.92, 95% CI 0.62 to 1.36; 264 participants; 2 trials; I2 = 0%), or polyethylene glycol versus lactulose (RR 0.57, 95% CI 0.18 to 1.82; 190 participants; 3 trials; I2 = 0%). Likewise, eight trials, involving 782 participants, reported a total of 374 non-serious adverse events and again our analyses found no beneficial or harmful effects of the pharmacotherapies under review when compared to placebo or to lactulose/lactitol.Nine trials, involving 733 participants, reported data on blood ammonia. We observed significant reductions in blood ammonia in placebo-controlled trials evaluating sodium benzoate (MD -32.00, 95% CI -46.85 to -17.15; 16 participants; 1 trial), glycerol phenylbutyrate (MD -12.00, 95% CI -23.37 to -0.63; 178 participants; 1 trial), ornithine phenylacetate (MD -27.10, 95% CI -48.55 to -5.65; 231 participants; 1 trial), and AST-120 (MD -22.00, 95% CI -26.75 to -17.25; 98 participants; 1 trial). However, there were no significant differences in blood ammonia concentrations in comparison with lactulose/lactitol with sodium benzoate (MD 9.00, 95% CI -1.10 to 19.11; 85 participants; 2 trials; I2 = 0%), AST-120 (MD 5.20, 95% CI -2.75 to 13.15; 35 participants; 1 trial), and polyethylene glycol (MD -29.28, 95% CI -95.96 to 37.39; 90 participants; 2 trials; I2 = 88%).
Funding: Five trials received support from pharmaceutical companies while four did not; two did not provide this information.
Authors' conclusions: There is insufficient evidence to determine the effects of these pharmacotherapies on the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. They have the potential to reduce blood ammonia concentrations when compared to placebo, but their overall effects on clinical outcomes of interest and the potential harms associated with their use remain uncertain. Further evidence is needed to evaluate the potential beneficial and harmful effects of these pharmacotherapies in this clinical setting.
Conflict of interest statement
Harry Zacharias: none Antony Zacharias: none Lise L Gluud: none Marsha Y Morgan: none
Figures





Update of
- doi: 10.1002/14651858.CD012334
References
References to studies included in this review
-
- Bajaj JS, Sheikh MY, Chojkier M, Balart L, Sherker AH, Vemuru R, et al. AST‐120 (spherical carbon adsorbent) in covert hepatic encephalopathy: results of the Astute trial. Journal of Hepatology 2013;58(Suppl 1):S84. [DOI: 10.1016/S0168-8278(13)60192-0] - DOI
- Bajaj JS, Sheikh MY, Chojkier M, Balart LA, Sherker AH, Vemuru RP, et al. Su1685 AST‐120 (spherical carbon adsorbent) in covert hepatic encephalopathy: results of the Astute trial. Gastroenterology 2013;144(5 (Suppl 1)):S997.
-
- Gonzalez A, Neri M, Campollo O, Amacio O. Treatment of acute portal systemic encephalopathy (PSE) grades III and IV with sodium benzoate and lactose. Hepatology (Baltimore, Md.) 1994;19(4 Suppl):67I.
-
- Pockros P, Hassanein T, Vierling J, Heuman D, Hillebrand D, Chojkier M. Phase 2, multicenter, randomised study of AST‐120 (spherical carbon adsorbent) vs. lactulose in the treatment of low‐grade hepatic encephalopathy (HE). Journal of Hepatology2009; Vol. 50, issue Suppl 1:S43. [CN‐00715816]
-
- Rahimi R, Singal A, Cuthbert J, Rockey D. Lactulose vs polyethylene glycol 3350‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomised clinical trial. Journal of the American Medical Association Internal Medicine 2014;174(11):1727‐33. [PUBMED: 25243839] - PMC - PubMed
- Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. A randomised trial of polyethylene glycol 3350‐electrolyte solution (PEG) and lactulose for patients hospitalized with acute hepatic encephalopathy. Hepatology (Baltimore, Md.) 2012;56(Suppl):915A‐6A.
References to studies excluded from this review
-
- Campollo O, Gil S, Olvera G, Poo JL, Uribe M. Efficacy and safety of sodium benzoate for the long‐term treatment of chronic hyperammonemic hepatic encephalopathy. Hepatology (Baltimore, Md.) 1992;16(Suppl 1):248A.
-
- Ghabril M, Zupanets I, Vierling J, Mantry P, Rockey D, Wolf D, et al. Glycerol phenylbutyrate in patients with cirrhosis and episodic hepatic encephalopathy: a pilot study of safety and effect on venous ammonia concentration. Clinical Pharmacology in Drug Development 2013;2(3):278‐84. [PUBMED: 27121790] - PubMed
-
- Mendenhall CL, Rouster S, Marshall L, Weesner R. A new therapy for portal systemic encephalopathy. American Journal of Gastroenterology 1986;81(7):540‐3. [PUBMED: 3717115] - PubMed
-
- Panella C, Guglielmi FW, Mastronuzzi T, Contento F, Siciliano N, Francavilla A. Oral Na‐benzoate in the treatment of chronic hepatic encephalopathy. Italian Journal of Gastroenterology 1993;25:228.
- Panella C, Guglielmi FW, Mastronuzzi T, Contento F, Siciliano N, Francavilla A. Oral sodium benzoate in the treatment of chronic hepatic encephalopathy. Gut 1993;34(Suppl 3):S43.
-
- Tripon S, Mallet M, Lodey M, Guiller E, Rudler M, Mouri S, et al. Sodium phenylbutyrate administration to avoid neurological worsening in cirrhotic patients with hepatic encephalopathy admitted in ICU. Journal of Hepatology 2016;64(2 Suppl):S288.
- Weiss N, Tripon S, Lodey M, Guiller E, Junot H, Monneret D, et al. Treating hepatic encephalopathy in cirrhotic patients admitted to ICU with sodium phenylbutyrate: a preliminary study. Fundamental & Clinical Pharmacology 2018;32(2):206‐8. - PubMed
References to ongoing studies
-
- NCT00558038. Safety and efficacy of AST‐120 compared to lactulose in patients with hepatic encephalopathy (AST015). clinicaltrials.gov/ct2/show/NCT00558038 (first posted 14 November 2007).
-
- NCT03448770. To compare efficacy and safety of lactulose versus polyethylene glycol for treatment of overt hepatic encephalopathy in cirrhotics: a randomised controlled trial. clinicaltrials.gov/ct2/show/nct03448770 (first posted 28 February 2018).
Additional references
-
- American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Journal of Hepatology 2014;61(3):642‐59. - PubMed
-
- Acharya C, Bajaj J. Current management of hepatic encephalopathy. American Journal of Gastroenterology 2018;113(11):1600‐12. - PubMed
-
- Ahuja N, Ally W, Caldwell S. Direct acting inhibitors of ammoniagenesis: a role in post‐TIPS encephalopathy?. Annals of Hepatology 2014;13(2):179‐86. - PubMed
-
- Allampati S, Duarte‐Rojo A, Thacker L, Patidar K, White M, Klair J, et al. Diagnosis of minimal hepatic encephalopathy using strop EncephalApp: a multicentre US‐based, norm‐based study. American Journal of Gastroenterology 2016;111(1):78‐86. [PUBMED: 26644276] - PubMed
-
- Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 2008;135(5):1591‐600. [PUBMED: 18723018] - PubMed
References to other published versions of this review
-
- Zacharias HD, Zacharias AP, Oliveira Ferreira A, Morgan MY, Gluud LL. Ammonia scavenging agents for people with cirrhosis and hepatic encephalopathy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012334] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

