Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure
- PMID: 31205027
- PMCID: PMC6597232
- DOI: 10.1172/JCI123825
Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure
Abstract
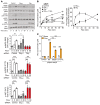
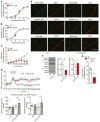
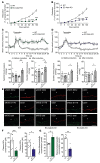
Hypertension is a primary risk factor for cardiovascular diseases including myocardial infarction and stroke. Major determinants of blood pressure are vasodilatory factors such as nitric oxide (NO) released from the endothelium under the influence of fluid shear stress exerted by the flowing blood. Several endothelial signaling processes mediating fluid shear stress-induced formation and release of vasodilatory factors have been described. It is, however, still poorly understood how fluid shear stress induces these endothelial responses. Here we show that the endothelial mechanosensitive cation channel PIEZO1 mediated fluid shear stress-induced release of adrenomedullin, which in turn activated its Gs-coupled receptor. The subsequent increase in cAMP levels promoted the phosphorylation of endothelial NO synthase (eNOS) at serine 633 through protein kinase A (PKA), leading to the activation of the enzyme. This Gs/PKA-mediated pathway synergized with the AKT-mediated pathways leading to eNOS phosphorylation at serine 1177. Mice with endothelium-specific deficiency of adrenomedullin, the adrenomedullin receptor, or Gαs showed reduced flow-induced eNOS activation and vasodilation and developed hypertension. Our data identify fluid shear stress-induced PIEZO1 activation as a central regulator of endothelial adrenomedullin release and establish the adrenomedullin receptor and subsequent Gs-mediated formation of cAMP as a critical endothelial mechanosignaling pathway regulating basal endothelial NO formation, vascular tone, and blood pressure.
Keywords: G-protein coupled receptors; Hypertension; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

