New biologics and small molecules in inflammatory bowel disease: an update
- PMID: 31205488
- PMCID: PMC6537282
- DOI: 10.1177/1756284819853208
New biologics and small molecules in inflammatory bowel disease: an update
Abstract
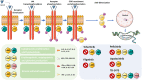
Inflammatory bowel disease (IBD) is a spectrum of immune-mediated inflammatory disorders with a complex multifactorial pathogenesis, where different pathways may predominate in different individuals. This complexity will most likely require a panoply of drugs targeting different pathways if one wants to treat to steroid-free sustained remission and mucosal healing. Presently, the mainstay of medical management of IBD is based on 5-aminosalicylates, corticosteroids, thiopurines, methotrexate, antitumor necrosis factor, anti-alpha4 beta7 (α4β7) integrin and anti-interleukin (IL)-12/IL-23 therapies. The discovery of new pathways involved in the pathogenesis of IBD resulted in new drugs targeting Janus kinase/signal transducers and activators of transcription, IL-6, spingosine-1-phosphate, and phosphodiesterase 4, among others. These new therapies might result in more advantageous safety profiles. Several of these new drugs have already been successfully tested in other inflammatory disorders, such as psoriasis or rheumatoid arthritis. In this review, evidence from phase II and phase III randomized controlled clinical trials in patients with IBD involving new biologicals and small molecules are summarized.
Keywords: Crohn’s disease; JAK inhibitor; S1P modulator; anti-integrin; biologicals; inflammatory bowel disease; small molecules; therapy; ulcerative colitis.
Conflict of interest statement
Conflict of interest statement: João Sabino has received speaker fees from AbbVie and Nestle Health Sciences. Bram Verstockt has received financial support for research from Pfizer; lecture fees from AbbVie, Ferring, Takeda Pharmaceuticals, Janssen and R-Biopharm AG; consultancy fees from Janssen. Marc Ferrante has received research grants from Janssen, Pfizer, and Takeda; consultancy fees from AbbVie, Boehringer-Ingelheim, Falk, Ferring, Janssen, Mitsubishi Tanabe, MSD, and Pfizer; speakers fees from AbbVie, Boehringer-Ingelheim, Chiesi, Falk, Ferring, Janssen, Mitsubishi Tanabe, MSD, Pfizer, and Tramedico. Séverine Vermeire has received grant support from AbbVie, MSD, Pfizer, J&J, and Takeda; received speaker fees from AbbVie, MSD, Takeda, Ferring, Dr. Falk Pharma, Hospira, Pfizer Inc., and Tillots; and served as a consultant for AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Robarts Clinical Trials, Gilead, Celgene, Prometheus, Avaxia, Prodigest, Shire, Pfizer Inc, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, and Janssen.
Figures

References
-
- De Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. - PubMed
-
- Ott C, Scholmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol 2013; 10: 585–595. - PubMed
-
- Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013; 145: 158–165.e152. - PubMed
-
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42; quiz e30. - PubMed
-
- Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

