Management of metastatic cutaneous melanoma: updates in clinical practice
- PMID: 31205512
- PMCID: PMC6535734
- DOI: 10.1177/1758835919851663
Management of metastatic cutaneous melanoma: updates in clinical practice
Abstract
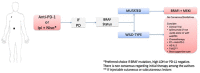
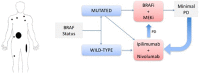
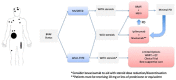
In recent years, several drugs have been approved for the treatment of patients with metastatic cutaneous melanoma, completely reshaping the landscape of this aggressive disease. Immune therapy with cytotoxic T-lymphocyte antigen 4 and programmed cell death-1 inhibitors yielded significant and durable responses, achieving long-term disease control in up to 40% of the patients. BRAF inhibitors (BRAFi), in combination with MEK inhibitors, also resulted in improved overall survival compared with single-agent BRAFi in patients with BRAFV600-mutated metastatic melanoma. The optimized sequencing and duration of treatment, however, is yet to be found. In this article, we thoroughly review current data and discuss how to best sequence the various treatment modalities available at present, based on four distinct clinical presentations commonly seen in clinic. In addition, we review treatment options beyond checkpoint inhibitors and targeted therapy, which may be required by patients failing such effective treatments.
Keywords: BRAF inhibitor; anti-PD-1; combination immunotherapy; immunotherapy; melanoma; metastatic brain tumors; targeted therapy; treatment sequencing.
Conflict of interest statement
Conflict of interest statement: Antonio C. Buzaid has served on an advisory board for MSD, BMS, Roche, AstraZeneca, Novartis, Pfizer, Eisai, and Blau. Michael B. Atkins has served on an advisory board for BMS, MSD, Roche, Novartis, Pfizer, Array, Esai, and Exelixis. Gustavo Schvartsman has performed a consulting role for BMS, United Medical, and MSD. Isabella C. Glitza, Sanjiv S. Agarwala, and Patricia Taranto have no conflicts of interest to disclose.
Figures

References
-
- Middleton MR, Grob J, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18: 158–158. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

