Removal of Surface Oxygen Vacancies Increases Conductance Through TiO2 Thin Films for Perovskite Solar Cells
- PMID: 31205577
- PMCID: PMC6559051
- DOI: 10.1021/acs.jpcc.9b02371
Removal of Surface Oxygen Vacancies Increases Conductance Through TiO2 Thin Films for Perovskite Solar Cells
Abstract
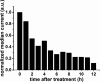
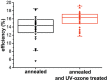
We report that UV-ozone treatment of TiO2 anatase thin films is an efficient method to increase the conductance through the film by more than 2 orders of magnitude. The increase in conductance is quantified via conductive scanning force microscopy on freshly annealed and UV-ozone-treated TiO2 anatase thin films on fluorine-doped tin oxide substrates. The increased conductance of TiO2 anatase thin films results in a 2% increase of the average power conversion efficiency (PCE) of methylammonium lead iodide-based perovskite solar cells. PCE values up to 19.5% for mesoporous solar cells are realized. The additional UV-ozone treatment results in a reduced number of oxygen vacancies at the surface, inferred from X-ray photoelectron spectroscopy. These oxygen vacancies at the surface act as charge carrier traps and hinder charge extraction from the adjacent material. Terahertz measurements indicate only minor changes of the bulk conductance, which underlines the importance of UV-ozone treatment to control surface-based defects.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Fathil M. F. M.; Adzhri R.; Arshad M. K. M.; Hashim U.; Ruslinda A. R.; Ayub R. M.; Nuzaihan M. N. M.; Azman A. H.; Zaki M. In Preparation and Characterization of Titanium Dioxide Thin Film for Field-Effect Transistor Biosensor Application, 2015 2nd International Conference on Biomedical Engineering (ICoBE), 2015; pp 30–31.
-
- Li M.; Li X.; Li W.; Meng X.; Yu Y.; Sun X. Atomic Layer Deposition Derived Amorphous TiO2 Thin Film Decorating Graphene Nanosheets with Superior Rate Capability. Electrochem. Commun. 2015, 57, 43–47. 10.1016/j.elecom.2015.05.005. - DOI
-
- Dongale T. D.; Shinde S. S.; Kamat R. K.; Rajpure K. Y. Nanostructured TiO2 Thin Film Memristor Using Hydrothermal Process. J. Alloys Compd. 2014, 593, 267–270. 10.1016/j.jallcom.2014.01.093. - DOI
-
- Yu J.; Zhao X.; Zhao Q. Effect of Surface Structure on Photocatalytic Activity of TiO2 Thin Films Prepared by Sol-Gel Method. Thin Solid Films 2000, 379, 7–14. 10.1016/S0040-6090(00)01542-X. - DOI
LinkOut - more resources
Full Text Sources
