Application of PD-1 Blockade in Cancer Immunotherapy
- PMID: 31205619
- PMCID: PMC6558092
- DOI: 10.1016/j.csbj.2019.03.006
Application of PD-1 Blockade in Cancer Immunotherapy
Abstract
The programmed cell death protein 1 (PD-1) pathway has received considerable attention due to its role in eliciting the immune checkpoint response of T cells, resulting in tumor cells capable of evading immune surveillance and being highly refractory to conventional chemotherapy. Application of anti-PD-1/PD-L1 antibodies as checkpoint inhibitors is rapidly becoming a promising therapeutic approach in treating tumors, and some of them have successfully been commercialized in the past few years. However, not all patients show complete responses and adverse events have been noted, suggesting a better understanding of PD-1 pathway mediated immunosuppression is needed to predict patient response and improve treatment efficacy. Here, we review the progresses on the studies of the mechanistic role of PD-1 pathway in the tumor immune evasion, recent clinical development and commercialization of PD-1 pathway inhibitors, the toxicities associated with PD-1 blockade observed in clinical trials as well as how to improve therapeutic efficacy and safety of cancer immunotherapy.
Keywords: 5-AZA-dC, 5-aza-2′-deoxycytidine; ADCC, Antibody-dependent cellular cytotoxicity; AEs, Adverse events; AP1, Activator protein 1; APCs, Antigen presenting cells; ASCT, Autologous stem cell transplantation; B2M, β2 microglobulin; BATF, Basic leucine zipper transcriptional factor ATF-like; BICR, Blinded Independent Central Review; BV, Brentuximab vedotin; CC, Cervical cancer; CRC, Colorectal cancer; CTLA-4, Cytotoxic T-lymphocyte–associated antigen 4; CXCL9, C-X-C motif chemokine ligand 9; Checkpoint blockade; DCM, Dilated cardiomyopathy; DCs, Dendritic cells; DNMT, DNA methyltransferase; DOR, Duration overall response; DZNep, 3-Deazaneplanocin A; ERK, Extracellular signal–regulated kinase; EZH2, Enhancer of zeste homolog 2; GC, Gastric cancer; GEJ, GASTRIC or gastroesophageal junction; HCC, Hepatocellular carcinoma; HNSCC, Head and neck squamous cell carcinoma; HR, Hazard ratio; ICC, Investigator-choice chemotherapy; ICOS, Inducible T-cell co-stimulator; IFN, Interferon; IHC, Immunohistochemistry; ITIM, Immune-receptortyrosine-based inhibitory motif; ITSM, Immune-receptortyrosine-based switch motif; ITT, Intention-to-treat; Immune surveillance; Immunotherapy; IrAEs, Immune related adverse events; JMJD3, Jumonji Domain-Containing Protein 3; LAG3, Lymphocyte-activation gene 3; LCK, Tyrosine-protein kinase Lck; MAP, Mitogen-activated protein; MCC, Merkel cell carcinoma; MHC, Major histocompatibility; MSI-H, Microsatellite instability-high; NF-κB, Nuclear factor-κB; NFAT, Nuclear factor of activated T cells; NSCLC, Non-small cell lung cancer; ORR, Overall response rate; OS, Overall survival; PD-1; PD-1, Programmed cell death 1; PD-L1; PD-L1, Programmed death-ligand 1; PFS, Progression-free survival; PI3K, Phosphoinositide 3-kinase; PKC, Protein kinase C; PMBCL, Primary mediastinal large B-cell lymphoma; PRC2, Polycomb repressive complex 2; PTEN, Phosphatase and tensin homolog; PTPs, Protein tyrosine phosphatases; RCC, Renal cell carcinoma; SCLC, Small cell lung cancer; SHP2, Src homology 2 domain-containing phosphatase 2; SIRPα, Signal-regulatory protein alpha; TCR, T-cell receptor; TGF, Transforming growth factor; TIICs, Tumor infiltrating immune cells; TILs, Tumor-infiltrating lymphocytes; TIM3, T-cell immunoglobulin and mucin-domain containing-3; TMB, Tumor mutation burden; TME, Tumor microenvironment; UC, Urothelial carcinoma; VEGF, Vascular endothelial growth factor; ZAP70, Zeta-chain-associated protein kinase 70; cHL, Classical Hodgkin lymphoma; cTnI, Cardiac troponin I; dMMR, DNA mismatch repair deficiency.
Figures

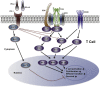
References
-
- Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- Parish C.R. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81:106–113. - PubMed
-
- Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
