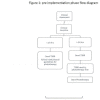
Quality improvement initiative using transcutaneous bilirubin nomogram to decrease serum bilirubin sampling in low-risk babies
- PMID: 31206073
- PMCID: PMC6542442
- DOI: 10.1136/bmjpo-2018-000403
Quality improvement initiative using transcutaneous bilirubin nomogram to decrease serum bilirubin sampling in low-risk babies
Abstract
Background: Screening for neonatal hyperbilirubinaemia in the postnatal ward has traditionally been performed using serum bilirubin sampling, but this has significant drawbacks such as risk of infection and slower reporting time.
Objective: We aimed to assess the impact of introducing transcutaneous bilirubin (TcBR) testing using TcBR nomogram on the number of serum bilirubin samples sent.
Methods: A before-and-after study was performed following the introduction of a protocol integrating the use of the Dragger JM-105 transcutaneous bilirubinometer in the postnatal ward. Only babies born at ≥37 weeks of gestation, weighing ≥2500 g who presented with jaundice after the first 24 hours and within the first 7 days of life were included in the study. The number of total serum bilirubin samples (TSBRs) sent were compared for the 6-month periods before and after (a total of 12 months) implementation of the new protocol.
Results: In the pre-implementation phase, a total of 882 (49%) out of 1815 babies had at least one serum bilirubin sample taken as opposed to a total of 236 (17%) out of 1394 babies in the post-implementation phase. The odds of performing TSBRs at least one time among babies in post-implementation phase were 79% lower than in pre-implementation phase (OR 0.21, 95% CI 0.18 to 0.25). We also estimated a significant cost saving of approximately US$1800 over a period of 6 months.
Conclusion: TcBR testing used in conjunction with our proposed nomogram significantly reduces the need for serum bilirubin sampling.
Keywords: TcB nomogram; jaundice; neonate.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effectiveness of transcutaneous bilirubin measurement in managing neonatal jaundice in postnatal ward of a tertiary care hospital in Pakistan.BMJ Paediatr Open. 2017 Aug 31;1(1):e000065. doi: 10.1136/bmjpo-2017-000065. eCollection 2017. BMJ Paediatr Open. 2017. PMID: 29637112 Free PMC article.
-
Implementation of a neonatal transcutaneous bilirubin screening programme in rural India.Paediatr Int Child Health. 2016 May;36(2):122-6. doi: 10.1179/2046905515Y.0000000013. Paediatr Int Child Health. 2016. PMID: 25844503
-
Validation of transcutaneous bilirubin nomogram in identifying neonates not at risk of hyperbilirubinaemia: a prospective, observational, multicenter study.Early Hum Dev. 2012 Jan;88(1):51-5. doi: 10.1016/j.earlhumdev.2011.07.001. Epub 2011 Jul 23. Early Hum Dev. 2012. PMID: 21782360
-
Evaluation of transcutaneous bilirubinometer (DRAEGER JM 103) use in Zimbabwean newborn babies.Matern Health Neonatol Perinatol. 2018 Jan 18;4:1. doi: 10.1186/s40748-017-0070-0. eCollection 2018. Matern Health Neonatol Perinatol. 2018. PMID: 29375886 Free PMC article.
-
Hyperbilirubinemia and transcutaneous bilirubinometry.Clin Chem. 2009 Jul;55(7):1280-7. doi: 10.1373/clinchem.2008.121889. Epub 2009 May 14. Clin Chem. 2009. PMID: 19443565 Review.
Cited by
-
The Impact of Phototherapy on the Accuracy of Transcutaneous Bilirubin Measurements in Neonates: Optimal Measurement Site and Timing.Diagnostics (Basel). 2021 Sep 20;11(9):1729. doi: 10.3390/diagnostics11091729. Diagnostics (Basel). 2021. PMID: 34574069 Free PMC article.
-
Comparing Kramer's rule with transcutaneous bilirubin vs. Kramer's rule only in reducing total serum bilirubin sampling among neonates with jaundice.BMC Pediatr. 2025 Mar 6;25(1):169. doi: 10.1186/s12887-025-05423-z. BMC Pediatr. 2025. PMID: 40045227 Free PMC article.
-
Effectiveness of Transcutaneous Bilirubin Measurement in High-Risk Neonates and to Evaluate Validity of Transcutaneous Bilirubin With Total Serum Bilirubin Levels in Both Low and High-Risk Neonates at a Tertiary Care Center in a Developing Country.Cureus. 2021 Mar 4;13(3):e13685. doi: 10.7759/cureus.13685. Cureus. 2021. PMID: 33833911 Free PMC article.
-
Management of neonatal jaundice in low- and lower-middle-income countries.BMJ Paediatr Open. 2019 Feb 14;3(1):e000408. doi: 10.1136/bmjpo-2018-000408. eCollection 2019. BMJ Paediatr Open. 2019. PMID: 30957028 Free PMC article. No abstract available.
References
-
- Begum NA, Alam K, Shaha A, et al. . Transcutaneous billirubinometry: a useful screening tool for neonatal jaundice in term and near term babies - a hospital based study. Bangladesh Journal of Child Health 2017;39:116–22. 10.3329/bjch.v39i3.31575 - DOI
-
- De Luca D, Zecca E, Zuppa AA, et al. . The joint use of human and electronic eye: visual assessment of jaundice and transcutaneous bilirubinometry. Turk J Pediatr 2008;50:456–61. - PubMed
LinkOut - more resources
Full Text Sources