Long-term Cognitive Outcomes in Patients With Pediatric-Onset vs Adult-Onset Multiple Sclerosis
- PMID: 31206130
- PMCID: PMC6580443
- DOI: 10.1001/jamaneurol.2019.1546
Long-term Cognitive Outcomes in Patients With Pediatric-Onset vs Adult-Onset Multiple Sclerosis
Abstract
Importance: Cognitive impairment in multiple sclerosis (MS) can lead to reduced quality of life, social functioning, and employment. Few studies have investigated cognitive outcomes among patients with pediatric-onset MS (POMS) over the long term.
Objective: To compare long-term information-processing efficiency between patients with POMS and adult-onset MS (AOMS).
Design, setting, and participants: This population-based longitudinal cohort study accessed the Swedish MS Registry (SMSreg), which collates information from all 64 neurology clinics in Sweden. Registered cases with definite MS in the SMSreg with an onset before April 15, 2018, and at least 2 Symbol Digit Modalities Test (SDMT) scores recorded were included. Only persons aged 18 to 55 years and with duration of disease of less than 30 years at the time of SDMT administration were included, to ensure comparable ranges between patients with POMS and AOMS. Of 8247 persons with an SDMT recorded in the SMSreg, 5704 met inclusion criteria, 300 (5.3%) of whom had POMS. Data were collected from April 1, 2006, through April 15, 2018 and analyzed from April through August 2018.
Exposures: Pediatric-onset MS (onset <18 years of age) vs AOMS (onset ≥18 years of age).
Main outcomes and measures: Information-processing efficiency measured every 6 or 12 months by the SDMT. Linear mixed-effects models were used to compare all available SDMT scores between patients with POMS and those with AOMS. Persons with cognitive impairment (ever vs never) were identified using regression-based norms and compared between POMS and AOMS groups using logistic regression.
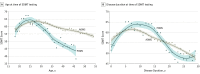
Results: Of the 5704 participants, 4015 were female (70.4%), and 5569 had a relapsing-onset disease course (97.6%). Most participants were exposed to a disease-modifying therapy (DMT) during follow-up (98.8%). Median age at baseline for the POMS group was 25.6 years (interquartile range, 21.0-31.7 years) and for the AOMS group, 38.3 years (interquartile range, 31.4-45.2 years). A total of 46 429 unique SDMT scores were analyzed. After adjustment for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and DMT exposure, the SDMT score for patients with POMS was significantly lower than that of patients with AOMS (β coefficient, -3.59 [95% CI, -5.56 to -1.54]). The SDMT score for patients with POMS declined faster than that of patients with AOMS (β coefficient, -0.30 [95% CI, -0.42 tp -0.17]). The odds of cognitive impairment were also significantly elevated in the POMS group (odds ratio, 1.44; 95% CI, 1.06-1.98).
Conclusions and relevance: In adulthood, patients with POMS demonstrated a more rapid reduction in information-processing efficiency over time and were more likely to experience cognitive impairment than patients with AOMS, independent of age or disease duration. Further investigation is required to understand the mechanisms by which early MS onset influences cognitive outcomes.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamaneurol.2019.0847
References
LinkOut - more resources
Full Text Sources
Miscellaneous

