Simvastatin Improves Neutrophil Function and Clinical Outcomes in Pneumonia. A Pilot Randomized Controlled Clinical Trial
- PMID: 31206313
- PMCID: PMC6857486
- DOI: 10.1164/rccm.201812-2328OC
Simvastatin Improves Neutrophil Function and Clinical Outcomes in Pneumonia. A Pilot Randomized Controlled Clinical Trial
Abstract
Rationale: Population studies suggest improved sepsis outcomes with statins, but the results of randomized controlled trials in patients with sepsis and organ dysfunction in critical care settings have broadly been negative. In vitro data suggest that statins modulate age-related neutrophil functions, improving neutrophil responses to infection, but only in older patients and at high doses.Objectives: To determine if high-dose simvastatin improves neutrophil functions and is safe and tolerated in hospitalized older adults with community-acquired pneumonia with sepsis (CAP + S) not admitted to critical care.Methods: We conducted a randomized, double-blind, placebo-controlled pilot study of simvastatin 80 mg or placebo for 7 days for patients with CAP + S aged 55 years or older admitted to a secondary care hospital. The Day 4 primary endpoint was change in neutrophil extracellular trap formation (NETosis). Day 4 secondary endpoints included neutrophil chemotaxis, safety and tolerability, Sequential Organ Failure Assessment score, mortality, readmission, and markers of tissue degradation/inflammation.Measurements and Main Results: Four days of simvastatin adjuvant therapy in patients with CAP + S was associated with improvements in systemic neutrophil function (NETosis and chemotaxis), a reduction in systemic neutrophil elastase burden, and improved Sequential Organ Failure Assessment scores compared with placebo. A post hoc analysis demonstrated that simvastatin therapy was associated with improved hospitalization-free survival compared with placebo. Simvastatin was well tolerated in this elderly and multimorbid patient group with common coprescription of macrolide antibiotics.Conclusions: This pilot study supports high-dose simvastatin as an adjuvant therapy for CAP + S in an older and milder disease cohort than assessed previously. A definitive multicenter study is now warranted in this population to assess the likelihood of benefit and harm.Clinical trial registered with EudraCT (2012-00343-29).
Keywords: elderly care; innate immunity; pneumonia; sepsis; statin.
Figures
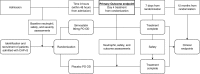
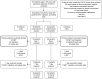
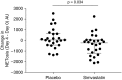
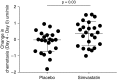


Comment in
-
Should We Tip Our CAPs to Statins?Am J Respir Crit Care Med. 2019 Nov 15;200(10):1204-1206. doi: 10.1164/rccm.201907-1295ED. Am J Respir Crit Care Med. 2019. PMID: 31310563 Free PMC article. No abstract available.
References
-
- Klapdor B, Ewig S, Schaberg T, Rohde G, Pletz MW, Schütte H, et al. CAPNETZ study group. Presentation, etiology and outcome of pneumonia in younger nursing home residents. J Infect. 2012;65:32–38. - PubMed
-
- Sapey E, Stockley RA. Red, amber and green: the role of the lung in de-priming active systemic neutrophils. Thorax. 2014;69:606–608. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

