HPV-negative Tumors in a Swedish Cohort of Cervical Cancer
- PMID: 31206367
- PMCID: PMC7147426
- DOI: 10.1097/PGP.0000000000000612
HPV-negative Tumors in a Swedish Cohort of Cervical Cancer
Abstract
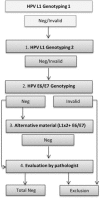
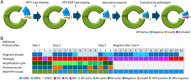
Despite the common perception that the human papilloma virus (HPV) is a requirement for the development of cervical cancer (CC), a considerable number of CCs test HPV negative. Presently, many countries are shifting to HPV primary CC screening, and it is of importance to increase the knowledge about the group of CCs that test HPV negative. The aim of this study was to reinvestigate a proportion of cervical tumors with a primary negative or invalid test result. Reinvestigation with repeated genotyping (targeting L1) was followed by analysis with an alternative target method (targeting E6/E7) on existing or additional tumor material. Consistently negative tumors were histologically evaluated, and cases with low or lacking tumor cell content, consistent invalid test results, or with suspicion of other than cervical origin were excluded. HPV-negative cases were thereafter subjected to immunohistochemistry (Cytokeratin 5, pan cytokeratin, protein 63, P16, and P53). The HPV-negative proportion could after reinvestigation be reduced by one-half (14%-7%). Additional positive samples were often detected in late polymerase chain reaction cycles, with an alternative (E6/E7) or the same (L1) target, or with a method using shorter amplicon lengths. Confirmed HPV negativity was significantly associated with worse prognosis, high patient age, longer storage time, and adenocarcinoma histology. Some of the HPV-negative cases showed strong/diffuse p16 immunoreactivity, indicating some remaining false-negative cases. False HPV negativity in this cohort was mainly linked to methodological limitations in the analysis of stored CC material. The small proportion of presumably true HPV-negative adenocarcinomas is not a reason for hesitation in revision to CC screening with primary HPV testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. - PubMed
-
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–56. - PubMed
-
- Harima Y, Sawada S, Nagata K, et al. Human papilloma virus (HPV) DNA associated with prognosis of cervical cancer after radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1345–51. - PubMed
-
- Rodriguez-Carunchio L, Soveral I, Steenbergen RD, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG 2015;122:119–27. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

