Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates
- PMID: 31206510
- PMCID: PMC6597128
- DOI: 10.1371/journal.pbio.3000328
Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates
Abstract
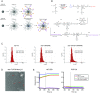
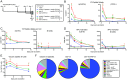
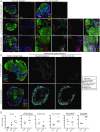
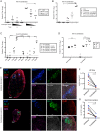
Peptide immunogens provide an approach to focus antibody responses to specific neutralizing sites on the HIV envelope protein (Env) trimer or on other pathogens. However, the physical characteristics of peptide immunogens can limit their pharmacokinetic and immunological properties. Here, we have designed synthetic "star" nanoparticles based on biocompatible N-[(2-hydroxypropyl)methacrylamide] (HPMA)-based polymer arms extending from a poly(amidoamine) (PAMAM) dendrimer core. In mice, these star nanoparticles trafficked to lymph nodes (LNs) by 4 hours following vaccination, where they were taken up by subcapsular macrophages and then resident dendritic cells (DCs). Immunogenicity optimization studies revealed a correlation of immunogen density with antibody titers. Furthermore, the co-delivery of Env variable loop 3 (V3) and T-helper peptides induced titers that were 2 logs higher than if the peptides were given in separate nanoparticles. Finally, we performed a nonhuman primate (NHP) study using a V3 glycopeptide minimal immunogen that was structurally optimized to be recognized by Env V3/glycan broadly neutralizing antibodies (bnAbs). When administered with a potent Toll-like receptor (TLR) 7/8 agonist adjuvant, these nanoparticles elicited high antibody binding titers to the V3 site. Similar to human V3/glycan bnAbs, certain monoclonal antibodies (mAbs) elicited by this vaccine were glycan dependent or targeted the GDIR peptide motif. To improve affinity to native Env trimer affinity, nonhuman primates (NHPs) were boosted with various SOSIP Env proteins; however, significant neutralization was not observed. Taken together, this study provides a new vaccine platform for administration of glycopeptide immunogens for focusing immune responses to specific bnAb epitopes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
HIV-1 Cross-Reactive Primary Virus Neutralizing Antibody Response Elicited by Immunization in Nonhuman Primates.J Virol. 2017 Oct 13;91(21):e00910-17. doi: 10.1128/JVI.00910-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835491 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Immunogenicity of a Prefusion HIV-1 Envelope Trimer in Complex with a Quaternary-Structure-Specific Antibody.J Virol. 2015 Dec 30;90(6):2740-55. doi: 10.1128/JVI.02380-15. J Virol. 2015. PMID: 26719262 Free PMC article.
-
HIV-1 immunogens and strategies to drive antibody responses towards neutralization breadth.Retrovirology. 2018 Nov 26;15(1):74. doi: 10.1186/s12977-018-0457-7. Retrovirology. 2018. PMID: 30477581 Free PMC article. Review.
-
Progress in HIV-1 vaccine development.Curr Opin HIV AIDS. 2013 Jul;8(4):326-32. doi: 10.1097/COH.0b013e328361d178. Curr Opin HIV AIDS. 2013. PMID: 23743722 Free PMC article. Review.
Cited by
-
Synthesis and Structure Optimization of Star Copolymers as Tunable Macromolecular Carriers for Minimal Immunogen Vaccine Delivery.Bioconjug Chem. 2024 Aug 21;35(8):1218-1232. doi: 10.1021/acs.bioconjchem.4c00273. Epub 2024 Jul 31. Bioconjug Chem. 2024. PMID: 39081220 Free PMC article.
-
Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies.Cell. 2021 May 27;184(11):2955-2972.e25. doi: 10.1016/j.cell.2021.04.042. Epub 2021 May 20. Cell. 2021. PMID: 34019795 Free PMC article.
-
The Impact of Sustained Immunization Regimens on the Antibody Response to Oligomannose Glycans.ACS Chem Biol. 2020 Mar 20;15(3):789-798. doi: 10.1021/acschembio.0c00053. Epub 2020 Mar 9. ACS Chem Biol. 2020. PMID: 32109354 Free PMC article.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
-
Dendritic Cells in HIV/SIV Prophylactic and Therapeutic Vaccination.Viruses. 2019 Dec 24;12(1):24. doi: 10.3390/v12010024. Viruses. 2019. PMID: 31878130 Free PMC article. Review.
References
-
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194(12):1661–71. 10.1086/508748 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

