Aggrecan is required for chondrocyte differentiation in ATDC5 chondroprogenitor cells
- PMID: 31206541
- PMCID: PMC6576788
- DOI: 10.1371/journal.pone.0218399
Aggrecan is required for chondrocyte differentiation in ATDC5 chondroprogenitor cells
Abstract
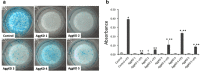
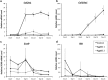
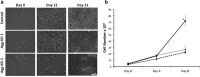
Aggrecan is an integral component of the extracellular matrix in cartilaginous tissues, including the growth plate. Heterozygous defects in the aggrecan gene have been identified as a cause of autosomal dominant short stature, bone age acceleration, and premature growth cessation. The mechanisms accounting for this phenotype remain unknown. We used ATDC5 cells, an established model of chondrogenesis, to evaluate the effects of aggrecan deficiency. ATDC5 aggrecan knockdown cell lines (AggKD) were generated using lentiviral shRNA transduction particles. Cells were stimulated with insulin/transferrin/selenium for up to 21 days to induce chondrogenesis. Control ATDC5 cells showed induction of Col2a1 starting at day 8 and induction of Col10a1 starting at day 12. AggKD cells had significantly reduced expression of Col2a1 and Col10a1 (p<0.0001) with only minimal increases in expression over time, indicating that chondrogenesis was markedly impaired. The induction of Col2a1 and Col10a1 was not rescued by culturing of AggKD cells in wells pre-conditioned with ATDC5 extracellular matrix or in co-culture with wild-type ATDC5 cells. We interpret our studies as indicating that aggrecan has an integral role in chondrogenesis that may be mediated through intracellular mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Vinculin functions as regulator of chondrogenesis.J Biol Chem. 2012 May 4;287(19):15760-75. doi: 10.1074/jbc.M111.308072. Epub 2012 Mar 13. J Biol Chem. 2012. PMID: 22416133 Free PMC article.
-
ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes.Bone. 2004 Jul;35(1):96-107. doi: 10.1016/j.bone.2004.03.015. Bone. 2004. PMID: 15207745
-
Ascorbate-enhanced chondrogenesis of ATDC5 cells.Eur Cell Mater. 2006 Nov 9;12:64-9; discussion 69-70. doi: 10.22203/ecm.v012a08. Eur Cell Mater. 2006. PMID: 17096313
-
The role of semaphorin 3A on chondrogenic differentiation.In Vitro Cell Dev Biol Anim. 2024 Jun;60(6):609-615. doi: 10.1007/s11626-024-00909-z. Epub 2024 May 10. In Vitro Cell Dev Biol Anim. 2024. PMID: 38727898 Free PMC article.
-
Stimulation of chondrogenesis in ATDC5 chondroprogenitor cells and hypertrophy in mouse by Genkwadaphnin.Eur J Pharmacol. 2011 Mar 25;655(1-3):9-15. doi: 10.1016/j.ejphar.2011.01.012. Epub 2011 Jan 23. Eur J Pharmacol. 2011. PMID: 21266170
Cited by
-
Selective inhibition of progesterone receptor in osteochondral progenitor cells, but not in mature chondrocytes, modulated subchondral bone structures.Bone. 2020 Mar;132:115196. doi: 10.1016/j.bone.2019.115196. Epub 2019 Dec 19. Bone. 2020. PMID: 31863959 Free PMC article.
-
Effects of self-assembled type II collagen fibrils on the morphology and growth of pre-chondrogenic ATDC5 cells.Osteoarthr Cartil Open. 2024 Feb 27;6(2):100450. doi: 10.1016/j.ocarto.2024.100450. eCollection 2024 Jun. Osteoarthr Cartil Open. 2024. PMID: 38444516 Free PMC article.
-
Callus organoids reveal distinct cartilage to bone transition mechanisms across donors and a role for biological sex.Bone Res. 2025 Mar 26;13(1):41. doi: 10.1038/s41413-025-00418-z. Bone Res. 2025. PMID: 40140357 Free PMC article.
-
Direct Scaffold-Coupled Electrical Stimulation of Chondrogenic Progenitor Cells through Graphene Foam Bioscaffolds to Control the Mechanical Properties of Graphene Foam-Cell Composites.ACS Appl Mater Interfaces. 2025 Jul 2;17(26):37404-37420. doi: 10.1021/acsami.5c02628. Epub 2025 May 20. ACS Appl Mater Interfaces. 2025. PMID: 40392077 Free PMC article.
-
Phenotypic Characterization of Immortalized Chondrocytes from a Desbuquois Dysplasia Type 1 Mouse Model: A Tool for Studying Defects in Glycosaminoglycan Biosynthesis.Int J Mol Sci. 2021 Aug 27;22(17):9304. doi: 10.3390/ijms22179304. Int J Mol Sci. 2021. PMID: 34502207 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

