Biomimetic Nanotechnology toward Personalized Vaccines
- PMID: 31206841
- PMCID: PMC6918015
- DOI: 10.1002/adma.201901255
Biomimetic Nanotechnology toward Personalized Vaccines
Abstract
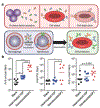
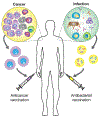
While traditional approaches for disease management in the era of modern medicine have saved countless lives and enhanced patient well-being, it is clear that there is significant room to improve upon the current status quo. For infectious diseases, the steady rise of antibiotic resistance has resulted in super pathogens that do not respond to most approved drugs. In the field of cancer treatment, the idea of a cure-all silver bullet has long been abandoned. As a result of the challenges facing current treatment and prevention paradigms in the clinic, there is an increasing push for personalized therapeutics, where plans for medical care are established on a patient-by-patient basis. Along these lines, vaccines, both against bacteria and tumors, are a clinical modality that could benefit significantly from personalization. Effective vaccination strategies could help to address many challenging disease conditions, but current vaccines are limited by factors such as a lack of potency and antigenic breadth. Recently, researchers have turned toward the use of biomimetic nanotechnology as a means of addressing these hurdles. Recent progress in the development of biomimetic nanovaccines for antibacterial and anticancer applications is discussed, with an emphasis on their potential for personalized medicine.
Keywords: anticancer vaccinations; bacteria vaccinations; biomimetic nanoparticles; nanomedicine; personalized medicine.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

