σ-Noninnocence: Masked Phenyl-Cation Transfer at Formal NiIV
- PMID: 31206937
- PMCID: PMC6771483
- DOI: 10.1002/anie.201906658
σ-Noninnocence: Masked Phenyl-Cation Transfer at Formal NiIV
Abstract
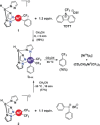
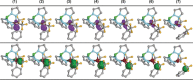
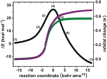
Reductive elimination is an elementary organometallic reaction step involving a formal oxidation state change of -2 at a transition-metal center. For a series of formal high-valent NiIV complexes, aryl-CF3 bond-forming reductive elimination was reported to occur readily (Bour et al. J. Am. Chem. Soc. 2015, 137, 8034-8037). We report a computational analysis of this reaction and find that, unexpectedly, the formal NiIV centers are better described as approaching a +II oxidation state, originating from highly covalent metal-ligand bonds, a phenomenon attributable to σ-noninnocence. A direct consequence is that the elimination of aryl-CF3 products occurs in an essentially redox-neutral fashion, as opposed to a reductive elimination. This is supported by an electron flow analysis which shows that an anionic CF3 group is transferred to an electrophilic aryl group. The uncovered role of σ-noninnocence in metal-ligand bonding, and of an essentially redox-neutral elimination as an elementary organometallic reaction step, may constitute concepts of broad relevance to organometallic chemistry.
Keywords: coinage metals; homolysis; ligand-field inversion; radicals; trifluoromethyl.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- None
-
- Terao J., Kambe N., Acc. Chem. Res. 2008, 41, 1545–1554; - PubMed
-
- Shiota H., Ano Y., Aihara Y., Fukumoto Y., Chatani N., J. Am. Chem. Soc. 2011, 133, 14952–14955; - PubMed
-
- Aihara Y., Chatani N., J. Am. Chem. Soc. 2013, 135, 5308–5311; - PubMed
-
- Aihara Y., Chatani N., J. Am. Chem. Soc. 2014, 136, 898–901; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

