Cortical and Commissural Defects Upon HCF-1 Loss in Nkx2.1-Derived Embryonic Neurons and Glia
- PMID: 31207118
- PMCID: PMC6771735
- DOI: 10.1002/dneu.22704
Cortical and Commissural Defects Upon HCF-1 Loss in Nkx2.1-Derived Embryonic Neurons and Glia
Abstract
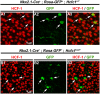
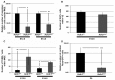
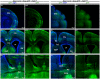
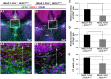
Formation of the cerebral cortex and commissures involves a complex developmental process defined by multiple molecular mechanisms governing proliferation of neuronal and glial precursors, neuronal and glial migration, and patterning events. Failure in any of these processes can lead to malformations. Here, we study the role of HCF-1 in these processes. HCF-1 is a conserved metazoan transcriptional co-regulator long implicated in cell proliferation and more recently in human metabolic disorders and mental retardation. Loss of HCF-1 in a subset of ventral telencephalic Nkx2.1-positive progenitors leads to reduced numbers of GABAergic interneurons and glia, owing not to decreased proliferation but rather to increased apoptosis before cell migration. The loss of these cells leads to development of severe commissural and cortical defects in early postnatal mouse brains. These defects include mild and severe structural defects of the corpus callosum and anterior commissure, respectively, and increased folding of the cortex resembling polymicrogyria. Hence, in addition to its well-established role in cell proliferation, HCF-1 is important for organ development, here the brain.
Keywords: GABAergic neurons; Nkx2.1; anterior commissure; corpus callosum; cortex; glia; polymicrogyria.
© 2019 The Authors. Developmental Neurobiology Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey.J Comp Neurol. 1990 Jan 22;291(4):520-37. doi: 10.1002/cne.902910404. J Comp Neurol. 1990. PMID: 2329189
-
Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain.Development. 2006 Jun;133(11):2243-52. doi: 10.1242/dev.02379. Development. 2006. PMID: 16690755
-
Midline radial glia translocation and corpus callosum formation require FGF signaling.Nat Neurosci. 2006 Jun;9(6):787-97. doi: 10.1038/nn1705. Epub 2006 May 21. Nat Neurosci. 2006. PMID: 16715082
-
Multiple non-cell-autonomous defects underlie neocortical callosal dysgenesis in Nfib-deficient mice.Neural Dev. 2009 Dec 4;4:43. doi: 10.1186/1749-8104-4-43. Neural Dev. 2009. PMID: 19961580 Free PMC article.
-
Cortical axon guidance by the glial wedge during the development of the corpus callosum.J Neurosci. 2001 Apr 15;21(8):2749-58. doi: 10.1523/JNEUROSCI.21-08-02749.2001. J Neurosci. 2001. PMID: 11306627 Free PMC article.
Cited by
-
Role of specialized composition of SWI/SNF complexes in prostate cancer lineage plasticity.Nat Commun. 2020 Nov 3;11(1):5549. doi: 10.1038/s41467-020-19328-1. Nat Commun. 2020. PMID: 33144576 Free PMC article.
-
Missense and nonsense mutations of the zebrafish hcfc1a gene result in contrasting mTor and radial glial phenotypes.Gene. 2023 May 15;864:147290. doi: 10.1016/j.gene.2023.147290. Epub 2023 Feb 17. Gene. 2023. PMID: 36804358 Free PMC article.
-
Hcfc1a regulates neural precursor proliferation and asxl1 expression in the developing brain.BMC Neurosci. 2020 Jun 10;21(1):27. doi: 10.1186/s12868-020-00577-1. BMC Neurosci. 2020. PMID: 32522152 Free PMC article.
-
Expression and regulation of SETBP1 in the song system of male zebra finches (Taeniopygia guttata) during singing.Sci Rep. 2024 Nov 23;14(1):29057. doi: 10.1038/s41598-024-75353-w. Sci Rep. 2024. PMID: 39580495 Free PMC article.
-
The role of HCFC1 in syndromic and non-syndromic intellectual disability.Med Res Arch. 2020 Jun;8(6):10.18103/mra.v8i6.2122. doi: 10.18103/mra.v8i6.2122. Epub 2020 Jun 18. Med Res Arch. 2020. PMID: 34164576 Free PMC article.
References
-
- Anderson, S.A. , Marin, O. , Horn, C. , Jennings, K. and Rubenstein, J.L. (2001) Distinct cortical migrations from the medial and lateral ganglionic eminences. Development, 128, 353–363. - PubMed
-
- Barkovich, A.J. , Kuzniecky, R.I. , Jackson, G.D. , Guerrini, R. and Dobyns, W.B. (2005) A developmental and genetic classification for malformations of cortical development. Neurology, 65, 1873–1887. - PubMed
-
- Bonneau, D. , Toutain, A. , Laquerriere, A. , Marret, S. , Saugier‐Veber, P. , Barthez, M.A. , et al (2002) X‐linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Annals of Neurology, 51, 340–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

