Low-Fat Diet Designed for Weight Loss But Not Weight Maintenance Improves Nitric Oxide-Dependent Arteriolar Vasodilation in Obese Adults
- PMID: 31207908
- PMCID: PMC6627594
- DOI: 10.3390/nu11061339
Low-Fat Diet Designed for Weight Loss But Not Weight Maintenance Improves Nitric Oxide-Dependent Arteriolar Vasodilation in Obese Adults
Abstract
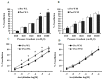
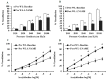
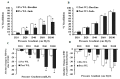
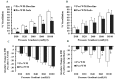
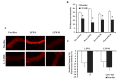
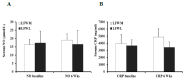
Obesity is associated with microvascular dysfunction. While low-fat diet improves cardiovascular risk, its contributions on microvascular function, independent of weight loss, is unknown. We tested the hypothesis that nitric oxide (NO)-dependent vasodilation in microvessels is improved by low-fat diets designed for weight loss (LFWL) compared to low-fat weight maintenance (LFWM) diet. Obese adults were randomly assigned to either a LFWL diet (n = 11) or LFWM diet (n = 10) for six weeks. Microvessels were obtained from gluteal subcutaneous fat biopsies before and after the intervention for vascular reactivity measurements to acetylcholine (Ach) and flow, with and without L-NAME or indomethacin. Vascular and serum NO and C-reactive protein (CRP) were also measured. LFWL diet increased flow-induced (FID) and ACh-induced dilation (AChID); an effect that was inhibited by L-NAME. Conversely, LFWM diet did not affect FID or AChID. Indomethacin improved FID and AChID in the baseline and this effect was minimized in response to both diets. Serum NO or CRP did not change in response to either diet. In conclusion, LFWL diet improves microvascular reactivity compared to LFWM diet and increased vascular NO contribution to the improved microvascular dilation. These data suggest that weight reduction on low fat diet is critical for microvascular health.
Keywords: acetylcholine; cardiovascular; flow-induced dilation; hypocaloric; isocaloric; low-fat diet; microvasculature; nitric oxide; obesity; weight loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients.Nutrients. 2019 Oct 18;11(10):2521. doi: 10.3390/nu11102521. Nutrients. 2019. PMID: 31635396 Free PMC article.
-
Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet.Am J Physiol Heart Circ Physiol. 2007 May;292(5):H2093-9. doi: 10.1152/ajpheart.01202.2006. Epub 2007 Jan 12. Am J Physiol Heart Circ Physiol. 2007. PMID: 17220180
-
MicroRNA-21 Contributes to Reduced Microvascular Function in Binge Drinking Young Adults.Alcohol Clin Exp Res. 2018 Feb;42(2):278-285. doi: 10.1111/acer.13565. Epub 2017 Dec 27. Alcohol Clin Exp Res. 2018. PMID: 29178290 Free PMC article.
-
Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity.Hypertension. 2008 Feb;51(2):376-82. doi: 10.1161/HYPERTENSIONAHA.107.101824. Epub 2008 Jan 14. Hypertension. 2008. PMID: 18195164 Free PMC article. Clinical Trial.
-
Fat-Specific Protein 27 Regulation of Vascular Function in Human Obesity.J Am Heart Assoc. 2019 Jun 4;8(11):e011431. doi: 10.1161/JAHA.118.011431. Epub 2019 Jun 1. J Am Heart Assoc. 2019. PMID: 31433737 Free PMC article.
Cited by
-
CD147 Levels in Blood and Adipose Tissues Correlate with Vascular Dysfunction in Obese Diabetic Adults.J Cardiovasc Dev Dis. 2021 Dec 28;9(1):7. doi: 10.3390/jcdd9010007. J Cardiovasc Dev Dis. 2021. PMID: 35050217 Free PMC article.
-
Obesity, Dietary Patterns, and Cardiovascular Disease: A Narrative Review of Metabolic and Molecular Pathways.Curr Issues Mol Biol. 2025 Jun 10;47(6):440. doi: 10.3390/cimb47060440. Curr Issues Mol Biol. 2025. PMID: 40699839 Free PMC article. Review.
-
Noncanonical Role of Telomerase in Regulation of Microvascular Redox Environment With Implications for Coronary Artery Disease.Function (Oxf). 2022 Sep 3;3(5):zqac043. doi: 10.1093/function/zqac043. eCollection 2022. Function (Oxf). 2022. PMID: 36168588 Free PMC article.
-
Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction.Biomedicines. 2021 Aug 18;9(8):1034. doi: 10.3390/biomedicines9081034. Biomedicines. 2021. PMID: 34440238 Free PMC article.
-
Differential responses of resistance arterioles to elevated intraluminal pressure in blacks and whites.Am J Physiol Heart Circ Physiol. 2021 Jul 1;321(1):H29-H37. doi: 10.1152/ajpheart.01023.2020. Epub 2021 May 21. Am J Physiol Heart Circ Physiol. 2021. PMID: 34018853 Free PMC article.
References
-
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016. National Center for Health Statistics; Hyattsville, MD, USA: 2017. pp. 1–8. NCHS Data Brief. - PubMed
-
- Krauss R.M., Eckel R.H., Howard B., Appel L.J., Daniels S.R., Deckelbaum R.J., Erdman J.W., Jr., Kris-Etherton P., Goldberg I.J., Kotchen T.A., et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.CIR.102.18.2284. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

