Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells
- PMID: 31207955
- PMCID: PMC6628373
- DOI: 10.3390/ijms20122915
Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells
Abstract
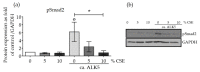
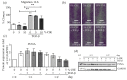
It is well established that smoking has detrimental effects on bone integrity and is a preventable risk factor for metabolic bone disorders. Following orthopedic surgeries, smokers frequently show delayed fracture healing associated with many complications, which results in prolonged hospital stays. One crucial factor responsible for fracture repair is the recruitment and differentiation of mesenchymal stem cells (MSCs) at early stages, a mechanism mediated by transforming growth factor β (TGF-β). Although it is known that smokers frequently have decreased TGF-β levels, little is known about the actual signaling occurring in these patients. We investigated the effect of cigarette smoke on TGF-β signaling in MSCs to evaluate which step in the pathway is affected by cigarette smoke extract (CSE). Single-cell-derived human mesenchymal stem cell line (SCP-1 cells) were treated with CSE concentrations associated with smoking up to 20 cigarettes a day. TGF-β signaling was analyzed using an adenovirus-based reporter assay system. Primary cilia structure and downstream TGF-β signaling modulators (Smad2, Smad3, and Smad4) were analyzed by Western blot and immunofluorescence staining. CSE exposure significantly reduced TGF-β signaling. Intriguingly, we observed that protein levels of phospho-Smad2/3 (active forms) as well as nuclear translocation of the phospho-Smad3/4 complex decreased after CSE exposure, phenomena that affected signal propagation. CSE exposure reduced the activation of TGF-β modulators under constitutive activation of TGF-β receptor type I (ALK5), evidencing that CSE affects signaling downstream of the ALK5 receptor but not the binding of the cytokine to the receptor itself. CSE-mediated TGF-β signaling impaired MSC migration, proliferation, and differentiation and ultimately affected endochondral ossification. Thus, we conclude that CSE-mediated disruption of TGF-β signaling in MSCs is partially responsible for delayed fracture healing in smokers.
Keywords: MSCs; Smad signaling; TGF-β signaling; bone metabolic diseases; cigarette smoke; fracture; osteoporosis; primary cilia; smokers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

