Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson's Disease and Its Inherent Risk for Melanoma
- PMID: 31208049
- PMCID: PMC6627191
- DOI: 10.3390/cells8060592
Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson's Disease and Its Inherent Risk for Melanoma
Abstract
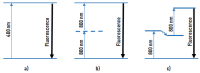
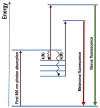
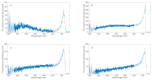
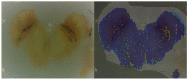
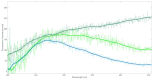
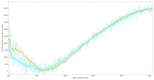
Parkinson's disease is associated with an increased risk of melanoma (and vice versa). Several hypotheses underline this link, such as pathways affecting both melanin and neuromelanin. For the first time, the fluorescence of melanin and neuromelanin is selectively accessible using a new method of nonlinear spectroscopy, based on a stepwise two-photon excitation. Cutaneous pigmentation and postmortem neuromelanin of Parkinson patients were characterized by fluorescence spectra and compared with controls. Spectral differences could not be documented, implying that there is neither a Parkinson fingerprint in cutaneous melanin spectra nor a melanin-associated fingerprint indicating an increased melanoma risk. Our measurements suggest that Parkinson's disease occurs without a configuration change of neuromelanin. However, Parkinson patients displayed the same dermatofluorescence spectroscopic fingerprint of a local malignant transformation as controls. This is the first comparative retrospective fluorescence analysis of cutaneous melanin and postmortem neuromelanin based on nonlinear spectroscopy in patients with Parkinson's disease and controls, and this method is a very suitable diagnostic tool for melanoma screening and early detection in Parkinson patients. Our results suggest a non-pigmentary pathway as the main link between Parkinson's disease and melanoma, and they do not rule out the melanocortin-1-receptor gene as an additional bridge between both diseases.
Keywords: Parkinson’s disease; dermatofluoroscopy; melanin; neuromelanin.
Conflict of interest statement
M.S. is the Managing Director of LTB, which developed the dermatofluoroscopy technique. L.S. is Head of Science, and G.S. is Head of Development in Magnosco GmbH, Berlin. No conflicts of interest were declared by D.L., P.R., S.S., C.-M.M., and H.-U.V.
Figures


Similar articles
-
New Aspects Regarding the Fluorescence Spectra of Melanin and Neuromelanin in Pigmented Human Tissue Concerning Hypoxia.Int J Mol Sci. 2024 Aug 2;25(15):8457. doi: 10.3390/ijms25158457. Int J Mol Sci. 2024. PMID: 39126026 Free PMC article.
-
Melanin and Neuromelanin: Linking Skin Pigmentation and Parkinson's Disease.Mov Disord. 2023 Feb;38(2):185-195. doi: 10.1002/mds.29260. Epub 2022 Nov 9. Mov Disord. 2023. PMID: 36350228
-
T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease.Mov Disord. 2011 Aug 1;26(9):1633-8. doi: 10.1002/mds.23722. Epub 2011 Apr 12. Mov Disord. 2011. PMID: 21491489
-
The enigma of neuromelanin in Parkinson's disease substantia nigra.J Neural Transm Suppl. 1994;43:113-22. J Neural Transm Suppl. 1994. PMID: 7884393 Review.
-
Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease.Prog Neurobiol. 2005 Feb;75(2):109-24. doi: 10.1016/j.pneurobio.2005.02.001. Prog Neurobiol. 2005. PMID: 15784302 Review.
Cited by
-
The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?Int J Mol Sci. 2024 Aug 3;25(15):8490. doi: 10.3390/ijms25158490. Int J Mol Sci. 2024. PMID: 39126061 Free PMC article.
-
Neuromelanin in Parkinson's Disease: Tyrosine Hydroxylase and Tyrosinase.Int J Mol Sci. 2022 Apr 10;23(8):4176. doi: 10.3390/ijms23084176. Int J Mol Sci. 2022. PMID: 35456994 Free PMC article. Review.
-
From Melanocytes to Melanoma Cells: Characterization of the Malignant Transformation by Four Distinctly Different Melanin Fluorescence Spectra (Review).Int J Mol Sci. 2021 May 17;22(10):5265. doi: 10.3390/ijms22105265. Int J Mol Sci. 2021. PMID: 34067690 Free PMC article. Review.
-
New Aspects Regarding the Fluorescence Spectra of Melanin and Neuromelanin in Pigmented Human Tissue Concerning Hypoxia.Int J Mol Sci. 2024 Aug 2;25(15):8457. doi: 10.3390/ijms25158457. Int J Mol Sci. 2024. PMID: 39126026 Free PMC article.
-
Perspective: Is a Closer Interaction between Experimental and Clinical Research Paradigms in Chronic Neurodegeneration, Such as Parkinson's Disease, Necessary Again?Cells. 2022 Dec 30;12(1):157. doi: 10.3390/cells12010157. Cells. 2022. PMID: 36611955 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

