Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration
- PMID: 31208051
- PMCID: PMC6632064
- DOI: 10.3390/polym11061048
Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration
Abstract
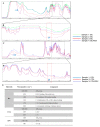
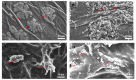
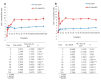
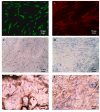
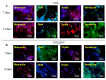
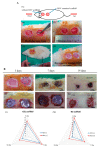
Excellent wound dressing is essential for effective wound repair and regeneration. However, natural polymeric skin substitutes often lack mechanical strength and hydrophilicity. One way to overcome this limitation is to use biodegradable polymers with high mechanical strength and low skin-irritation induction in wet environments. Bacterial cellulose (BC) is an attractive polymer for medical applications; unlike synthetic polymers, it is biodegradable and renewable and has a strong affinity for materials containing hydroxyl groups. Therefore, we conjugated it with resveratrol (RSV), which has a 4'-hydroxyl group and exhibits good biocompatibility and no cytotoxicity. We synthesized BC scaffolds with immobilized RSV and characterized the resulting BC/RSV scaffold with scanning electron microscopy and Fourier-transform infrared spectroscopy. We found that RSV was released from the BC in vitro after ~10 min, and immunofluorescence staining showed that BC was highly biocompatible and regenerated epithelia. Additionally, Masson's trichrome staining showed that the scaffolds preserved the normal collagen-bundling pattern and induced re-epithelialization in defective rat epidermis. These results indicated that RSV-conjugated BC created a biocompatible environment for stem cell attachment and growth and promoted epithelial regeneration during wound healing.
Keywords: bacterial cellulose; biodegradable polymer biomaterials; epidermal reconstruction; tissue engineering scaffolds; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Bacterial Cellulose as a Potential Bio-Scaffold for Effective Re-Epithelialization Therapy.Pharmaceutics. 2021 Sep 30;13(10):1592. doi: 10.3390/pharmaceutics13101592. Pharmaceutics. 2021. PMID: 34683885 Free PMC article.
-
Composite Scaffolds Based on Bacterial Cellulose for Wound Dressing Application.ACS Appl Bio Mater. 2022 Aug 15;5(8):3722-3733. doi: 10.1021/acsabm.2c00226. Epub 2022 Jul 19. ACS Appl Bio Mater. 2022. PMID: 35853242
-
Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering.Gels. 2023 Aug 14;9(8):656. doi: 10.3390/gels9080656. Gels. 2023. PMID: 37623111 Free PMC article.
-
Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing.Materials (Basel). 2023 Aug 3;16(15):5459. doi: 10.3390/ma16155459. Materials (Basel). 2023. PMID: 37570163 Free PMC article. Review.
-
Overview of bacterial cellulose composites: a multipurpose advanced material.Carbohydr Polym. 2013 Nov 6;98(2):1585-98. doi: 10.1016/j.carbpol.2013.08.018. Epub 2013 Aug 15. Carbohydr Polym. 2013. PMID: 24053844 Review.
Cited by
-
Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications.Int J Mol Sci. 2023 Jan 4;24(2):986. doi: 10.3390/ijms24020986. Int J Mol Sci. 2023. PMID: 36674505 Free PMC article. Review.
-
Resveratrol's bibliometric and visual analysis from 2014 to 2023.Front Plant Sci. 2024 Oct 8;15:1423323. doi: 10.3389/fpls.2024.1423323. eCollection 2024. Front Plant Sci. 2024. PMID: 39439517 Free PMC article.
-
The impact of resveratrol on skin wound healing, scarring, and aging.Int Wound J. 2022 Jan;19(1):9-28. doi: 10.1111/iwj.13601. Epub 2021 May 5. Int Wound J. 2022. PMID: 33949795 Free PMC article.
-
Bacterial Cellulose as a Potential Bio-Scaffold for Effective Re-Epithelialization Therapy.Pharmaceutics. 2021 Sep 30;13(10):1592. doi: 10.3390/pharmaceutics13101592. Pharmaceutics. 2021. PMID: 34683885 Free PMC article.
-
Evaluation of Different Methods for Cultivating Gluconacetobacter hansenii for Bacterial Cellulose and Montmorillonite Biocomposite Production: Wound-Dressing Applications.Polymers (Basel). 2020 Jan 26;12(2):267. doi: 10.3390/polym12020267. Polymers (Basel). 2020. PMID: 31991906 Free PMC article.
References
-
- Kucińska-Lipka J., Gubanska I., Janik H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015;72:2399–2419. doi: 10.1007/s00289-015-1407-3. - DOI
-
- Lee J.W., Deng F., Yeomans W.G., Allen A.L., Gross R.A., Kaplan D.L. Direct incorporation of glucosamine and n-acetylglucosamine into exopolymers by Gluconacetobacter xylinus (= Acetobacter xylinum) ATCC 10245: Production of chitosan-cellulose and chitin-cellulose exopolymers. Appl. Environ. Microbiol. 2001;67:3970–3975. doi: 10.1128/AEM.67.9.3970-3975.2001. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

