Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts
- PMID: 31208056
- PMCID: PMC6630352
- DOI: 10.3390/molecules24122239
Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts
Abstract
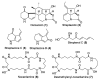
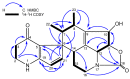
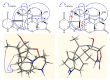
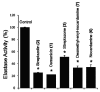
The strain Streptomyces osmaniensis CA-244599 isolated from the Comoros islands was submitted to liquid-state fermentation coupled to in situ solid-phase extraction with amberlite XAD-16 resin. Elution of the trapped compounds on the resin beads by ethyl acetate afforded seven metabolites, osmanicin (1), streptazolin (2), streptazone C (3), streptazone B1 (4), streptenol C (5), nocardamine (6) and desmethylenylnocardamine (7). Osmanicin (1) is a newly reported unusual scaffold combining streptazolin (2) and streptazone C (3) through a Diels-Alder type reaction. Experimental evidence excluded the spontaneous formation of 1 from 2 and 3. The isolated compounds were evaluated for their ability to inhibit elastase using normal human diploid fibroblasts. Compound 1 exhibited the most potent activity with an IC50 of 3.7 μM.
Keywords: Streptomyces osmaniensis; alkaloids; elastase inhibition; in situ solid-phase extraction; osmanicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


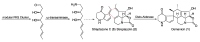

Similar articles
-
Identification and Characterization of the Streptazone E Biosynthetic Gene Cluster in Streptomyces sp. MSC090213JE08.Chembiochem. 2015 Nov 2;16(16):2385-91. doi: 10.1002/cbic.201500317. Epub 2015 Sep 25. Chembiochem. 2015. PMID: 26403163
-
Novel aromatic polyketides from soil Streptomyces spp.: purification, characterization and bioactivity studies.World J Microbiol Biotechnol. 2018 Apr 24;34(5):67. doi: 10.1007/s11274-018-2448-1. World J Microbiol Biotechnol. 2018. PMID: 29691661
-
HLE-inhibitory alkaloids with a polyketide skeleton from the marine-derived fungus Coniothyrium cereale.J Nat Prod. 2011 Oct 28;74(10):2282-5. doi: 10.1021/np2004227. Epub 2011 Sep 16. J Nat Prod. 2011. PMID: 21923104
-
Piperidine polyketide alkaloids of bacterial origin: Occurrence, bioactivity, and biosynthesis.Eur J Med Chem. 2025 May 5;289:117498. doi: 10.1016/j.ejmech.2025.117498. Epub 2025 Mar 10. Eur J Med Chem. 2025. PMID: 40088660 Review.
-
Dimeric naphthylisoquinoline alkaloids: polyketide-derived axially chiral bioactive quateraryls.Nat Prod Rep. 2019 Nov 1;36(11):1513-1545. doi: 10.1039/c9np00024k. Epub 2019 May 28. Nat Prod Rep. 2019. PMID: 31134266 Review.
Cited by
-
Combinatorial biosynthesis yields novel hybrid argimycin P alkaloids with diverse scaffolds in Streptomyces argillaceus.Microb Biotechnol. 2022 Dec;15(12):2905-2916. doi: 10.1111/1751-7915.14167. Epub 2022 Nov 8. Microb Biotechnol. 2022. PMID: 36346129 Free PMC article.
-
New Metabolites from the Marine Sponge Scopalina hapalia Collected in Mayotte Lagoon.Mar Drugs. 2022 Mar 2;20(3):186. doi: 10.3390/md20030186. Mar Drugs. 2022. PMID: 35323485 Free PMC article.
-
Solid-Phase Extraction Embedded Dialysis (SPEED), an Innovative Procedure for the Investigation of Microbial Specialized Metabolites.Mar Drugs. 2021 Jun 26;19(7):371. doi: 10.3390/md19070371. Mar Drugs. 2021. PMID: 34206861 Free PMC article.
-
Molecular characterization of the human kidney interstitium in health and disease.Sci Adv. 2021 Feb 10;7(7):eabd3359. doi: 10.1126/sciadv.abd3359. Print 2021 Feb. Sci Adv. 2021. PMID: 33568476 Free PMC article.
References
-
- Le Goff G., Martin M.-T., Iorga B.I., Adelin I., Servy C., Cortial S., Ouazzani J. Isolation and Characterization of Unusual Hydrazides from Streptomyces sp. Impact of the Cultivation Support and Extraction Procedure. J. Nat. Prod. 2013;76:142–149. - PubMed
-
- Georgousaki K., Katsinas N., Tsafantakis N., Gumeni S., Oves-Costales D., González I., Almeida C., Genilloud O., Trougakos I.N., Fokialakis N. Actinobacteria of global biodiversity as a source of bioactive metabolites for the discovery and development of novel cosmeuceutical agents. PMIO. 2017;4:S1–S202.

