Effect of Cyclic Stretch on Tissue Maturation in Myoblast-Laden Hydrogel Fibers
- PMID: 31208059
- PMCID: PMC6630375
- DOI: 10.3390/mi10060399
Effect of Cyclic Stretch on Tissue Maturation in Myoblast-Laden Hydrogel Fibers
Abstract
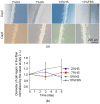
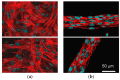
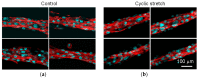
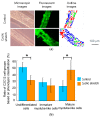
Engineering of the skeletal muscles has attracted attention for the restoration of damaged muscles from myopathy, injury, and extraction of malignant tumors. Reconstructing a three-dimensional muscle using living cells could be a promising approach. However, the regenerated tissue exhibits a weak construction force due to the insufficient tissue maturation. The purpose of this study is to establish the reconstruction system for the skeletal muscle. We used a cell-laden core-shell hydrogel microfiber as a three-dimensional culture to control the cellular orientation. Moreover, to mature the muscle tissue in the microfiber, we also developed a custom-made culture device for imposing cyclic stretch stimulation using a motorized stage and the fiber-grab system. As a result, the directions of the myotubes were oriented and the mature myotubes could be formed by cyclic stretch stimulation.
Keywords: core-shell hydrogel fiber; cyclic stretch; engineered muscle; myoblast; skeletal muscle.
Conflict of interest statement
H.O is a stockholder and a board member of Cellfiber Inc. which has licenses for certain cell fiber-related technologies and patents from The University of Tokyo.
Figures
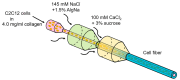


Similar articles
-
Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo.Biomaterials. 2017 Jul;131:98-110. doi: 10.1016/j.biomaterials.2017.03.026. Epub 2017 Mar 23. Biomaterials. 2017. PMID: 28388499
-
Rapid Fabrication of Cell-Laden Microfibers for Construction of Aligned Biomimetic Tissue.Front Bioeng Biotechnol. 2021 Jan 18;8:610249. doi: 10.3389/fbioe.2020.610249. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33585412 Free PMC article.
-
Bioreactor Platform for Biomimetic Culture and in situ Monitoring of the Mechanical Response of in vitro Engineered Models of Cardiac Tissue.Front Bioeng Biotechnol. 2020 Jul 14;8:733. doi: 10.3389/fbioe.2020.00733. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32766218 Free PMC article.
-
Nanofiber Yarn/Hydrogel Core-Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation.ACS Nano. 2015 Sep 22;9(9):9167-79. doi: 10.1021/acsnano.5b03644. Epub 2015 Aug 19. ACS Nano. 2015. PMID: 26280983
-
Extracellular Matrix-Derived Hydrogels as Biomaterial for Different Skeletal Muscle Tissue Replacements.Materials (Basel). 2020 May 29;13(11):2483. doi: 10.3390/ma13112483. Materials (Basel). 2020. PMID: 32486040 Free PMC article. Review.
Cited by
-
Microfluidic Formulation of Topological Hydrogels for Microtissue Engineering.Chem Rev. 2022 Nov 23;122(22):16839-16909. doi: 10.1021/acs.chemrev.1c00798. Epub 2022 Sep 15. Chem Rev. 2022. PMID: 36108106 Free PMC article. Review.
-
Combinatorial extracellular matrix cues with mechanical strain induce differential effects on myogenesis in vitro.Biomater Sci. 2023 Aug 22;11(17):5893-5907. doi: 10.1039/d3bm00448a. Biomater Sci. 2023. PMID: 37477446 Free PMC article.
-
Next Stage Approach to Tissue Engineering Skeletal Muscle.Bioengineering (Basel). 2020 Sep 30;7(4):118. doi: 10.3390/bioengineering7040118. Bioengineering (Basel). 2020. PMID: 33007935 Free PMC article. Review.
-
Editorial for the Special Issue of Selected Papers from the 9th Symposium on Micro-Nano Science and Technology on Micromachines.Micromachines (Basel). 2019 Sep 17;10(9):618. doi: 10.3390/mi10090618. Micromachines (Basel). 2019. PMID: 31533239 Free PMC article.
-
Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering.ACS Biomater Sci Eng. 2022 Feb 14;8(2):379-405. doi: 10.1021/acsbiomaterials.1c01145. Epub 2022 Jan 27. ACS Biomater Sci Eng. 2022. PMID: 35084836 Free PMC article. Review.
References
-
- Chimenti I., Gaetani R., Barile L., Forte E., Ionta V., Angelini F., Frati G., Messina E., Giacomello A. Isolation and Expansion of Adult Cardiac Stem/Progenitor Cells in the Form of Cardiospheres from Human Cardiac Biopsies and Murine Hearts. Humana Press; Totowa, NJ, USA: 2012. pp. 327–338. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

