The Biosynthesis, Signaling, and Neurological Functions of Bile Acids
- PMID: 31208099
- PMCID: PMC6628048
- DOI: 10.3390/biom9060232
The Biosynthesis, Signaling, and Neurological Functions of Bile Acids
Abstract
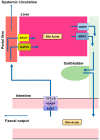
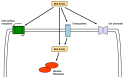
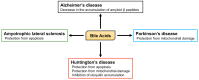
Bile acids (BA) are amphipathic steroid acids synthesized from cholesterol in the liver. They act as detergents to expedite the digestion and absorption of dietary lipids and lipophilic vitamins. BA are also considered to be signaling molecules, being ligands of nuclear and cell-surface receptors, including farnesoid X receptor and Takeda G-protein receptor 5. Moreover, BA also activate ion channels, including the bile acid-sensitive ion channel and epithelial Na+ channel. BA regulate glucose and lipid metabolism by activating these receptors in peripheral tissues, such as the liver and brown and white adipose tissue. Recently, 20 different BA have been identified in the central nervous system. Furthermore, BA affect the function of neurotransmitter receptors, such as the muscarinic acetylcholine receptor and γ-aminobutyric acid receptor. BA are also known to be protective against neurodegeneration. Here, we review recent findings regarding the biosynthesis, signaling, and neurological functions of BA.
Keywords: ALS; Alzheimer’s disease; FXR; Huntington’s disease; Parkinson’s disease; SHP; TGR5; bile acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Overview of Bile Acids Signaling and Perspective on the Signal of Ursodeoxycholic Acid, the Most Hydrophilic Bile Acid, in the Heart.Biomolecules. 2018 Nov 27;8(4):159. doi: 10.3390/biom8040159. Biomolecules. 2018. PMID: 30486474 Free PMC article. Review.
-
Bridging cell surface receptor with nuclear receptors in control of bile acid homeostasis.Acta Pharmacol Sin. 2015 Jan;36(1):113-8. doi: 10.1038/aps.2014.118. Epub 2014 Dec 15. Acta Pharmacol Sin. 2015. PMID: 25500873 Free PMC article. Review.
-
Advances in bile acid medicinal chemistry.Curr Med Chem. 2011;18(26):4029-52. doi: 10.2174/092986711796957266. Curr Med Chem. 2011. PMID: 21824088 Review.
-
Intestinal Farnesoid X Receptor and Takeda G Protein Couple Receptor 5 Signaling in Metabolic Regulation.Dig Dis. 2017;35(3):241-245. doi: 10.1159/000450981. Epub 2017 Mar 1. Dig Dis. 2017. PMID: 28249273 Free PMC article. Review.
-
Effects of bile acids on neurological function and disease.FASEB J. 2016 Nov;30(11):3658-3668. doi: 10.1096/fj.201600275R. Epub 2016 Jul 28. FASEB J. 2016. PMID: 27468758 Free PMC article. Review.
Cited by
-
The Role of Gut Microbiota-Derived Lithocholic Acid, Deoxycholic Acid and Their Derivatives on the Function and Differentiation of Immune Cells.Microorganisms. 2023 Nov 8;11(11):2730. doi: 10.3390/microorganisms11112730. Microorganisms. 2023. PMID: 38004742 Free PMC article. Review.
-
Lipids, Gut Microbiota, and the Complex Relationship with Alzheimer's Disease: A Narrative Review.Nutrients. 2023 Nov 3;15(21):4661. doi: 10.3390/nu15214661. Nutrients. 2023. PMID: 37960314 Free PMC article. Review.
-
Bile Acids Gate Dopamine Transporter Mediated Currents.Front Chem. 2021 Dec 10;9:753990. doi: 10.3389/fchem.2021.753990. eCollection 2021. Front Chem. 2021. PMID: 34957043 Free PMC article.
-
The Impact of Cholecystectomy on Colorectal Cancer Risk: A Comprehensive Review on Risk Factors and the Association.Curr Gastroenterol Rep. 2025 Aug 11;27(1):59. doi: 10.1007/s11894-025-01008-z. Curr Gastroenterol Rep. 2025. PMID: 40784992 Review.
-
Methylated Cytochrome P450 and the Solute Carrier Family of Genes Correlate With Perturbations in Bile Acid Metabolism in Parkinson's Disease.Front Neurosci. 2022 Mar 31;16:804261. doi: 10.3389/fnins.2022.804261. eCollection 2022. Front Neurosci. 2022. PMID: 35431771 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

