Involvement of E3 Ligases and Deubiquitinases in the Control of HIF-α Subunit Abundance
- PMID: 31208103
- PMCID: PMC6627837
- DOI: 10.3390/cells8060598
Involvement of E3 Ligases and Deubiquitinases in the Control of HIF-α Subunit Abundance
Abstract
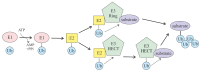
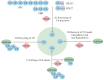
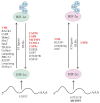
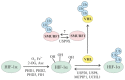
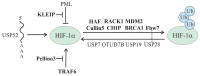
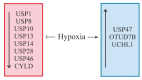
The ubiquitin and hypoxia-inducible factor (HIF) pathways are cellular processes involved in the regulation of a variety of cellular functions. Enzymes called ubiquitin E3 ligases perform protein ubiquitylation. The action of these enzymes can be counteracted by another group of enzymes called deubiquitinases (DUBs), which remove ubiquitin from target proteins. The balanced action of these enzymes allows cells to adapt their protein content to a variety of cellular and environmental stress factors, including hypoxia. While hypoxia appears to be a powerful regulator of the ubiquitylation process, much less is known about the impact of DUBs on the HIF system and hypoxia-regulated DUBs. Moreover, hypoxia and DUBs play crucial roles in many diseases, such as cancer. Hence, DUBs are considered to be promising targets for cancer cell-specific treatment. Here, we review the current knowledge about the role DUBs play in the control of HIFs, the regulation of DUBs by hypoxia, and their implication in cancer progression.
Keywords: DUBs; E3 ligases; HIF; Hypoxia; cancer; ubiquitylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
DUBs, Hypoxia, and Cancer.Trends Cancer. 2019 Oct;5(10):632-653. doi: 10.1016/j.trecan.2019.08.005. Epub 2019 Oct 11. Trends Cancer. 2019. PMID: 31706510 Review.
-
The intricate interplay between HIFs, ROS, and the ubiquitin system in the tumor hypoxic microenvironment.Pharmacol Ther. 2022 Dec;240:108303. doi: 10.1016/j.pharmthera.2022.108303. Epub 2022 Nov 1. Pharmacol Ther. 2022. PMID: 36328089 Review.
-
DUBs, New Members in the Hypoxia Signaling clUb.Front Oncol. 2016 Mar 9;6:53. doi: 10.3389/fonc.2016.00053. eCollection 2016. Front Oncol. 2016. PMID: 27014628 Free PMC article. Review.
-
Role of Protein Ubiquitination and HIF Signaling in the Evolution of Hypoxic Breast Cancer.Curr Pharm Biotechnol. 2024;25(17):2183-2185. doi: 10.2174/0113892010292219240212065544. Curr Pharm Biotechnol. 2024. PMID: 38409721 Review.
-
The functional interplay between the HIF pathway and the ubiquitin system - more than a one-way road.Exp Cell Res. 2017 Jul 15;356(2):152-159. doi: 10.1016/j.yexcr.2017.03.027. Epub 2017 Mar 15. Exp Cell Res. 2017. PMID: 28315321 Review.
Cited by
-
DDR1 Drives Malignant Progression of Gastric Cancer by Suppressing HIF-1α Ubiquitination and Degradation.Adv Sci (Weinh). 2024 Sep;11(35):e2308395. doi: 10.1002/advs.202308395. Epub 2024 Jul 18. Adv Sci (Weinh). 2024. PMID: 39024501 Free PMC article.
-
Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway.Int J Mol Sci. 2024 Dec 24;26(1):10. doi: 10.3390/ijms26010010. Int J Mol Sci. 2024. PMID: 39795869 Free PMC article.
-
UCHL1-dependent control of hypoxia-inducible factor transcriptional activity during liver fibrosis.Biosci Rep. 2024 Jun 26;44(6):BSR20232147. doi: 10.1042/BSR20232147. Biosci Rep. 2024. PMID: 38808772 Free PMC article.
-
Hypoxia Reduces Mouse Urine Output via HIF1α-Mediated Upregulation of Renal AQP1.Kidney Dis (Basel). 2024 Oct 22;10(6):504-518. doi: 10.1159/000542087. eCollection 2024 Dec. Kidney Dis (Basel). 2024. PMID: 39664329 Free PMC article.
-
Spring viremia of carp virus infection induces hypoxia response in zebrafish by stabilizing hif1α.J Virol. 2025 Jan 31;99(1):e0149124. doi: 10.1128/jvi.01491-24. Epub 2024 Nov 27. J Virol. 2025. PMID: 39601573 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

