Interactions of Glutamatergic Neurotransmission and Brain-Derived Neurotrophic Factor in the Regulation of Behaviors after Nicotine Administration
- PMID: 31208140
- PMCID: PMC6627482
- DOI: 10.3390/ijms20122943
Interactions of Glutamatergic Neurotransmission and Brain-Derived Neurotrophic Factor in the Regulation of Behaviors after Nicotine Administration
Abstract
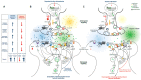
Nicotine causes tobacco dependence, which may result in fatal respiratory diseases. The striatum is a key structure of forebrain basal nuclei associated with nicotine dependence. In the striatum, glutamate release is increased when α7 nicotinic acetylcholine receptors expressed in the glutamatergic terminals are exposed to nicotine, and over-stimulates glutamate receptors in gamma amino-butyric acid (GABA)ergic neurons. These receptor over-stimulations in turn potentiate GABAergic outputs to forebrain basal nuclei and contribute to the increase in psychomotor behaviors associated with nicotine dependence. In parallel with glutamate increases, nicotine exposure elevates brain-derived neurotrophic factor (BDNF) release through anterograde and retrograde targeting of the synapses of glutamatergic terminals and GABAergic neurons. This article reviews nicotine-exposure induced elevations of glutamatergic neurotransmission, the bidirectional targeting of BDNF in the striatum, and the potential regulatory role played by BDNF in behavioral responses to nicotine exposure.
Keywords: TrkB; glutamate receptor; neurotrophic factor; nicotine dependence; striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function.J Neurosci. 2004 May 5;24(18):4340-50. doi: 10.1523/JNEUROSCI.0055-04.2004. J Neurosci. 2004. PMID: 15128848 Free PMC article.
-
Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons.J Neurosci. 2002 Sep 1;22(17):7580-5. doi: 10.1523/JNEUROSCI.22-17-07580.2002. J Neurosci. 2002. PMID: 12196581 Free PMC article.
-
Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins.J Neurosci. 2002 Sep 15;22(18):7903-12. doi: 10.1523/JNEUROSCI.22-18-07903.2002. J Neurosci. 2002. PMID: 12223543 Free PMC article.
-
Brain derived neurotrophic factor (BDNF), its tyrosine kinase receptor B (TrkB) and nicotine.Neurotoxicology. 2018 Mar;65:186-195. doi: 10.1016/j.neuro.2018.02.014. Epub 2018 Mar 2. Neurotoxicology. 2018. PMID: 29499216 Review.
-
Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review.
Cited by
-
Analysis of the brain transcriptome in lines of laying hens divergently selected for feather pecking.BMC Genomics. 2020 Aug 27;21(1):595. doi: 10.1186/s12864-020-07002-1. BMC Genomics. 2020. PMID: 32854615 Free PMC article.
-
Ras Inhibitor Lonafarnib Rescues Structural and Functional Impairments of Synapses of Aβ1-42 Mice via α7nAChR-Dependent BDNF Upregulation.J Neurosci. 2022 Aug 3;42(31):6090-6107. doi: 10.1523/JNEUROSCI.1989-21.2022. Epub 2022 Jun 27. J Neurosci. 2022. PMID: 35760529 Free PMC article.
-
Overnight Abstinence Is Associated With Smaller Secondary Somatosensory Cortical Volumes and Higher Somatosensory-Motor Cortical Functional Connectivity in Cigarette Smokers.Nicotine Tob Res. 2022 Nov 12;24(12):1889-1897. doi: 10.1093/ntr/ntac168. Nicotine Tob Res. 2022. PMID: 35796689 Free PMC article.
-
Simultaneous Intake of Chlorella and Ascidian Ethanolamine Plasmalogen Accelerates Activation of BDNF-TrkB-CREB Signaling in Rats.Molecules. 2024 Jan 11;29(2):357. doi: 10.3390/molecules29020357. Molecules. 2024. PMID: 38257270 Free PMC article.
-
Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex.Biomolecules. 2020 Jun 3;10(6):848. doi: 10.3390/biom10060848. Biomolecules. 2020. PMID: 32503154 Free PMC article.
References
-
- Capelletto E., Rapetti S.G., Demichelis S., Galetta D., Catino A., Ricci D., Moretti A.M., Bria E., Pilotto S., Bruno A., et al. Final data of an Italian multicentric survey about counseling for smoking cessation in patients with diagnosis of a respiratory disease. Clin. Respir. J. 2018;3:1150–1159. doi: 10.1111/crj.12644. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

