Chromosomal Evolution and Evolutionary Relationships of Lebiasina Species (Characiformes, Lebiasinidae)
- PMID: 31208145
- PMCID: PMC6628269
- DOI: 10.3390/ijms20122944
Chromosomal Evolution and Evolutionary Relationships of Lebiasina Species (Characiformes, Lebiasinidae)
Abstract
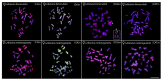
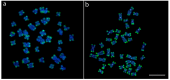
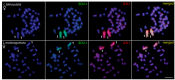
We present the first cytogenetic data for Lebiasina bimaculata and L. melanoguttata with the aim of (1) investigating evolutionary events within Lebiasina and their relationships with other Lebiasinidae genera and (2) checking the evolutionary relationships between Lebiasinidae and Ctenoluciidae. Both species have a diploid number 2n = 36 with similar karyotypes and microsatellite distribution patterns but present contrasting C-positive heterochromatin and CMA3+ banding patterns. The remarkable interstitial series of C-positive heterochromatin occurring in L. melanoguttata is absent in L. bimaculata. Accordingly, L. bimaculata shows the ribosomal DNA sites as the only GC-rich (CMA3+) regions, while L. melanoguttata shows evidence of a clear intercalated CMA3+ banding pattern. In addition, the multiple 5S and 18S rDNA sites in L. melanogutatta contrast with single sites present in L. bimaculata. Comparative genomic hybridization (CGH) experiments also revealed a high level of genomic differentiation between both species. A polymorphic state of a conspicuous C-positive, CMA3+, and (CGG)n band was found only to occur in L. bimaculata females, and its possible relationship with a nascent sex chromosome system is discussed. Whole chromosome painting (WCP) and CGH experiments indicate that the Lebiasina species examined and Boulengerella maculata share similar chromosomal sequences, thus supporting the relatedness between them and the evolutionary relationships between the Lebiasinidae and Ctenoluciidae families.
Keywords: comparative genomic hybridization; fish; karyotype evolution; whole chromosome painting.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Cytogenetics of the small-sized fish, Copeina guttata (Characiformes, Lebiasinidae): Novel insights into the karyotype differentiation of the family.PLoS One. 2019 Dec 19;14(12):e0226746. doi: 10.1371/journal.pone.0226746. eCollection 2019. PLoS One. 2019. PMID: 31856256 Free PMC article.
-
Evolutionary Relationships and Cytotaxonomy Considerations in the Genus Pyrrhulina (Characiformes, Lebiasinidae).Zebrafish. 2017 Dec;14(6):536-546. doi: 10.1089/zeb.2017.1465. Epub 2017 Aug 2. Zebrafish. 2017. PMID: 28767325
-
An Insight into the Chromosomal Evolution of Lebiasinidae (Teleostei, Characiformes).Genes (Basel). 2020 Mar 28;11(4):365. doi: 10.3390/genes11040365. Genes (Basel). 2020. PMID: 32231057 Free PMC article.
-
Evolutionary Relationships among Boulengerella Species (Ctenoluciidae, Characiformes): Genomic Organization of Repetitive DNAs and Highly Conserved Karyotypes.Cytogenet Genome Res. 2017;152(4):194-203. doi: 10.1159/000480141. Epub 2017 Sep 23. Cytogenet Genome Res. 2017. PMID: 28942442
-
Chromosomal Analysis of Ctenolucius hujeta Valenciennes, 1850 (Characiformes): A New Piece in the Chromosomal Evolution of the Ctenoluciidae.Cytogenet Genome Res. 2021;161(3-4):195-202. doi: 10.1159/000515456. Epub 2021 Jun 14. Cytogenet Genome Res. 2021. PMID: 34126615
Cited by
-
Chromosomes of Asian cyprinid fishes: Novel insight into the chromosomal evolution of Labeoninae (Teleostei, Cyprinidae).PLoS One. 2024 Feb 7;19(2):e0292689. doi: 10.1371/journal.pone.0292689. eCollection 2024. PLoS One. 2024. PMID: 38324533 Free PMC article.
-
The Genetic Differentiation of Pyrrhulina (Teleostei, Characiformes) Species is Likely Influenced by Both Geographical Distribution and Chromosomal Rearrangements.Front Genet. 2022 May 4;13:869073. doi: 10.3389/fgene.2022.869073. eCollection 2022. Front Genet. 2022. PMID: 35601496 Free PMC article.
-
Cytogenetics Meets Genomics: Cytotaxonomy and Genomic Relationships among Color Variants of the Asian Arowana Scleropages formosus.Int J Mol Sci. 2023 May 19;24(10):9005. doi: 10.3390/ijms24109005. Int J Mol Sci. 2023. PMID: 37240350 Free PMC article.
-
Comparative study of four Mystus species (Bagridae, Siluriformes) from Thailand: insights into their karyotypic diversity.Comp Cytogenet. 2021 Apr 26;15(2):119-136. doi: 10.3897/CompCytogen.v15i2.60649. eCollection 2021. Comp Cytogenet. 2021. PMID: 33959235 Free PMC article.
-
Chromosomal mapping of repetitive DNA and retroelement sequences and its implications for the chromosomal evolution process in Ctenoluciidae (Characiformes).BMC Ecol Evol. 2024 May 30;24(1):72. doi: 10.1186/s12862-024-02262-x. BMC Ecol Evol. 2024. PMID: 38816840 Free PMC article.
References
-
- Weitzman M., Weitzman S.H. Check List of the Freshwater Fishes of South and Central America. Edipucrs; Porto Alegre, Brazil: 2003. Family Lebiasinidae; pp. 241–250.
-
- Fricke R., Eschmeyer W.N., van der Laan R. Catalog of Fishes: Genera, Species, References. [(accessed on 9 April 2019)]; Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishc....
-
- Weitzman S.H., Vari R.P. Miniaturization South American Fishes; An Overview and Discussion. Proc. Biol. Soc. Washingt. 1988;2:444–465.
-
- Netto-Ferreira A.L. Ph.D. Thesis. Universidade de São Paulo; São Paulo, Brazil: 2010. Revisão taxonômica e relações interespecíficas de Lebiasinidae (Ostariophysi: Characiformes: Lebiasinidae)
-
- Oyakawa O.T. Ph.D. Thesis. Universidade de Sao Paulo; Sao Paulo, Brazil: 1998. Relações filogenéticas das famílias Pyrrhulinidae, Lebiasinidae e Erythrinidae (Osteichthyes: Characiformes)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

