2-Iodo-4'-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway
- PMID: 31208152
- PMCID: PMC6631972
- DOI: 10.3390/molecules24122249
2-Iodo-4'-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway
Abstract
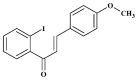
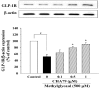
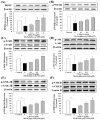
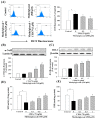
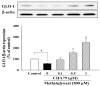
Methylglyoxal (MG) acts as a reactive precursor of advanced glycation end products (AGEs). This compound is often connected with pathologies such as diabetes, neurodegenerative processes and diseases of aging. 2-iodo-4'-methoxychalcone (CHA79), a synthetic halogen-containing chalcone derivative, has been reported its anti-diabetic activity. This study aims to investigate the potential protective capability of CHA79 against MG-mediated neurotoxicity in SH-SY5Y cells. Results indicated CHA79 increased viability of cells and attenuated the rate of apoptosis in MG-exposed SH-SY5Y. CHA79 up-regulated expression of anti-apoptotic protein (Bcl-2) and down-regulated apoptotic proteins (Bax, cytochrome c, caspase-3, caspase-9). Moreover, CHA79 significantly up-regulated expression of neurotrophic factors, including glucagon-like peptide-1 receptor (GLP-1R), brain derived neurotrophic factor (BDNF), p75NTR, p-TrkB, p-Akt, p-GK-3β and p-CREB. CHA79 attenuated MG-induced ROS production and enhanced the antioxidant defense including nuclear factor erythroid 2-related factor 2 (Nrf2), HO-1, SOD and GSH. Furthermore, CHA79 attenuated MG-induced reduction of glyoxalase-1 (GLO-1), a vital enzyme on removing AGE precursors. In conclusion, CHA79 is the first novel synthetic chalcone possessing the GLP-1R and GLO-1 activating properties. CHA 79 also exhibits neuroprotective effects against MG toxicity by enhancing neurotrophic signal, antioxidant defense and anti-apoptosis pathway.
Keywords: antioxidant defense; glyoxalase pathway; halogen-containing chalcones; methylglyoxal; neuroprotection; neurotrophic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Antioxidant, Anti-Inflammatory, and Neuroprotective Properties of the Synthetic Chalcone Derivative AN07.Molecules. 2020 Jun 24;25(12):2907. doi: 10.3390/molecules25122907. Molecules. 2020. PMID: 32599797 Free PMC article.
-
Naringenin, a dietary flavanone, enhances insulin-like growth factor 1 receptor-mediated antioxidant defense and attenuates methylglyoxal-induced neurite damage and apoptotic death.Nutr Neurosci. 2021 Jan;24(1):71-81. doi: 10.1080/1028415X.2019.1594554. Epub 2019 Mar 22. Nutr Neurosci. 2021. PMID: 30900959
-
Neuroprotective Effects of a Combination of Dietary Trans-Resveratrol and Hesperidin Against Methylglyoxal-Induced Neurotoxicity in a Depressive Amnesia Mouse Model.Nutrients. 2025 Apr 30;17(9):1548. doi: 10.3390/nu17091548. Nutrients. 2025. PMID: 40362855 Free PMC article.
-
The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders.Curr Protein Pept Sci. 2020;21(9):846-859. doi: 10.2174/1389203721666200505101734. Curr Protein Pept Sci. 2020. PMID: 32368974 Review.
-
Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway.Redox Biol. 2018 Apr;14:465-473. doi: 10.1016/j.redox.2017.10.015. Epub 2017 Oct 18. Redox Biol. 2018. PMID: 29080525 Free PMC article. Review.
Cited by
-
Design, synthesis, docking, ADMET and anticancer evaluations of N-alkyl substituted iodoquinazoline derivatives as dual VEGFR-2 and EGFR inhibitors.RSC Adv. 2023 Dec 13;13(51):36301-36321. doi: 10.1039/d3ra07700d. eCollection 2023 Dec 8. RSC Adv. 2023. PMID: 38093733 Free PMC article.
-
Effect and mechanism of BDNF/TrkB signaling on vestibular compensation.Bioengineered. 2021 Dec;12(2):11823-11836. doi: 10.1080/21655979.2021.1997565. Bioengineered. 2021. PMID: 34719333 Free PMC article.
-
Anti-Glucotoxicity Effect of Phytoconstituents via Inhibiting MGO-AGEs Formation and Breaking MGO-AGEs.Int J Mol Sci. 2023 Apr 21;24(8):7672. doi: 10.3390/ijms24087672. Int J Mol Sci. 2023. PMID: 37108833 Free PMC article. Review.
-
Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia.Antioxidants (Basel). 2022 Nov 17;11(11):2275. doi: 10.3390/antiox11112275. Antioxidants (Basel). 2022. PMID: 36421461 Free PMC article. Review.
-
Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity.Antioxidants (Basel). 2020 Aug 3;9(8):697. doi: 10.3390/antiox9080697. Antioxidants (Basel). 2020. PMID: 32756399 Free PMC article.
References
-
- Lo T.W., Westwood M.E., McLellan A.C., Selwood T., Thornalley P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994;269:32299–32305. - PubMed
-
- Shimatani Y., Nodera H., Osaki Y., Banzrai C., Takayasu K., Endo S., Shibuta Y., Kaji R. Upregulation of axonal HCN current by methylglyoxal: Potential association with diabetic polyneuropathy. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015;126:2226–2232. doi: 10.1016/j.clinph.2015.02.058. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

