The Impact of Limbal Mesenchymal Stromal Cells on Healing of Acute Ocular Surface Wounds Is Improved by Pre-cultivation and Implantation in the Presence of Limbal Epithelial Cells
- PMID: 31208228
- PMCID: PMC6767890
- DOI: 10.1177/0963689719858577
The Impact of Limbal Mesenchymal Stromal Cells on Healing of Acute Ocular Surface Wounds Is Improved by Pre-cultivation and Implantation in the Presence of Limbal Epithelial Cells
Abstract
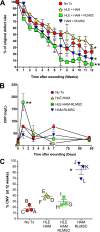
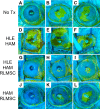
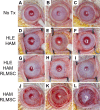
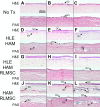
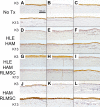
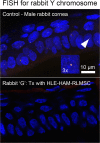
While limbal epithelial cells are used for treating ocular surface wounds, the therapeutic potential of mesenchymal cells cultivated from the limbal stroma (LMSC) is less clear. We have therefore examined the effects of LMSC when applied to acute ocular surface wounds. LMSC derived from male rabbits (RLMSC) were applied to the ocular surface of female rabbits immediately following removal of the corneal and limbal epithelium. Human amniotic membrane (HAM) was used as the vehicle for implanting the RLMSC. The effects of RLMSC were examined when applied alone (n = 3) and in conjunction with a stratified culture of human limbal epithelial cells (HLE) grown on the opposing surface of the HAM (n = 3). Outcomes were monitored over 3 months in comparison with animals receiving no treatment (n = 3) or treatment with HLE alone on HAM (n = 3). Animals treated with RLMSC (n = 6) displayed faster re-epithelialization (∼90% versus 70% healing after 12 weeks), with best results being observed when RLMSC were pre-cultivated and implanted in the presence of HLE (p < 0.01; 90% healing by 7 weeks). While all animals displayed conjunctival cells on the corneal surface (by presence of goblet cells and/or keratin 13 expression) and corneal neovascularization, evidence of corneal epithelial regeneration was observed in animals that received RLMSC in the presence of HLE (by staining for keratin 3 and the absence of goblet cells). Conversely, corneal neovascularization was significantly greater when RLMSC were applied in the absence of HLE (<0.05; 90% of cornea compared with 20-30% in other cohorts). Nevertheless, neither human nuclear antigen nor rabbit Y chromosome were detected within the regenerated epithelium. Our results demonstrate that while cultured LMSC encourage corneal re-epithelialization, healing is improved by the pre-cultivation and implantation of these mesenchymal cells in the presence of limbal epithelial cells.
Keywords: Algerbrush II; amniotic membrane; corneal neovascularization; limbal mesenchymal stromal cells; ocular surface; wound healing.
Conflict of interest statement
Figures


Similar articles
-
Evaluation of the AlgerBrush II rotating burr as a tool for inducing ocular surface failure in the New Zealand White rabbit.Exp Eye Res. 2016 Jun;147:1-11. doi: 10.1016/j.exer.2016.04.005. Epub 2016 Apr 13. Exp Eye Res. 2016. PMID: 27085211
-
A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction.Stem Cells Transl Med. 2015 Sep;4(9):1052-63. doi: 10.5966/sctm.2015-0039. Epub 2015 Jul 16. Stem Cells Transl Med. 2015. PMID: 26185258 Free PMC article.
-
Cultured corneal epithelia for ocular surface disease.Trans Am Ophthalmol Soc. 1999;97:891-986. Trans Am Ophthalmol Soc. 1999. PMID: 10703147 Free PMC article.
-
Chemical injuries of the eye: current concepts in pathophysiology and therapy.Surv Ophthalmol. 1997 Jan-Feb;41(4):275-313. doi: 10.1016/s0039-6257(96)00007-0. Surv Ophthalmol. 1997. PMID: 9104767 Review.
-
Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction.Eur J Ophthalmol. 2003 Jul;13(6):515-24. doi: 10.1177/112067210301300602. Eur J Ophthalmol. 2003. PMID: 12948308 Review.
Cited by
-
Immunophenotypical Characterization of Limbal Mesenchymal Stromal Cell Subsets during In Vitro Expansion.Int J Mol Sci. 2024 Aug 9;25(16):8684. doi: 10.3390/ijms25168684. Int J Mol Sci. 2024. PMID: 39201371 Free PMC article.
-
Biomedical Application of MSCs in Corneal Regeneration and Repair.Int J Mol Sci. 2025 Jan 15;26(2):695. doi: 10.3390/ijms26020695. Int J Mol Sci. 2025. PMID: 39859409 Free PMC article. Review.
-
TLR3-overexpressing umbilical cord mesenchymal stromal cells suppress immune responses to attenuate high-risk corneal transplantation rejection.Stem Cell Res Ther. 2025 Jul 15;16(1):370. doi: 10.1186/s13287-025-04510-3. Stem Cell Res Ther. 2025. PMID: 40660345 Free PMC article.
-
Potential of mesenchymal stem cells as topical immunomodulatory cell therapies for ocular surface inflammatory disorders.Stem Cells Transl Med. 2021 Jan;10(1):39-49. doi: 10.1002/sctm.20-0118. Epub 2020 Sep 8. Stem Cells Transl Med. 2021. PMID: 32896982 Free PMC article. Review.
-
Therapeutic effect of mesenchymal stem cells and their derived exosomes in diseases.Mol Biomed. 2025 Jun 4;6(1):34. doi: 10.1186/s43556-025-00277-4. Mol Biomed. 2025. PMID: 40465163 Free PMC article. Review.
References
-
- Holland EJ. Management of limbal stem cell deficiency: a historical perspective, past, present, and future. Cornea. 2015;34(Suppl 10):S9–S15. - PubMed
-
- Ainscough SL, Linn ML, Barnard Z, Schwab IR, Harkin DG. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp Eye Res. 2011;92(1):10–19. - PubMed
-
- O’Callaghan AR, Morgan L, Daniels JT, Lewis MP. Human-derived feeder fibroblasts for the culture of epithelial cells for clinical use. Regen Med. 2016;11(6):529–543. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

