EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer
- PMID: 31208370
- PMCID: PMC6580637
- DOI: 10.1186/s12885-019-5820-0
EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer
Abstract
Background: Epidermal growth factor receptor exon 20 insertion (EGFRex20ins) mutations represent approximately 4-12% of EGFR mutations and are generally refractory to the 1st and 2nd generation EGFR tyrosine kinase inhibitors (TKIs). Development of effective therapies for patients with EGFRex20ins mutant non-small-cell lung carcinoma (NSCLC) represents a great unmet need. Preclinical models have shown that osimertinib is active in NSCLC harboring EGFRex20ins, while the antitumor activity of osimertinib remains to be evaluated in patients with EGFRex20ins mutations.
Methods: Tumor genotyping was performed in 2316 Chinese NSCLC cases with targeted next generation sequencing (NGS) covering the whole exons of EGFR gene. The frequency and genetic characteristics of EGFRexon20ins mutations were analyzed. Furthermore, six patients with specific EGFRexon20ins mutations and receiving osimertinib 80 mg once daily were retrospectively included to assess the antitumor activity and safety of monotherapy osimertinib.
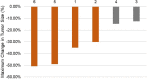
Results: EGFRex20ins mutations were identified in 4.8% (53/1095) of EGFR mutant NSCLC and 2.3% (53/2316) of all NSCLC cases. The most frequently identified EGFRexon20ins is A767_V769dup (17/53,32.1%). We found that the genetic characteristics of EGFRex20ins mutations in Chinese patients with NSCLC were comparable to those reported in Caucasian patients. Four patients with osimertinib therapy achieved partial response and the rest stable disease. Median progression free survival (PFS) was 6.2 months (95% confidence interval 5.0-12.9 months; range 4.9-14.6 months). The most common adverse events (AEs) were diarrhea (2/6), pruritis (2/6), stomatitis (1/6) and nausea (1/6). No grade 3 or more AEs were documented.
Conclusions: This study revealed that the genetic characteristics of EGFRex20ins mutations in Chinese patients with NSCLC were comparable to those reported in Caucasian patients. Furthermore, our study firstly demonstrated promising antitumor activity of osimertinib in certain EGFRex20ins mutant advanced NSCLC patients, indicating that osimertinib treatment for EGFRex20ins positive patients deserves further study.
Keywords: EGFRex20ins; NGS; NSCLC; Osimertinib.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama-J Am Med Assoc. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. - DOI - PMC - PubMed
-
- Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, Wu G, Liu W, Liao G, Cai K, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology - mainland China subset analysis of the PIONEER study. PLoS One. 2015;10(11):e0143515. doi: 10.1371/journal.pone.0143515. - DOI - PMC - PubMed
-
- Travis WD. WHO classification of the pathology and genetics of tumors of the lung. J Thorac Oncol. 2015;10(9):S68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

