Comparison of 0.05% cyclosporine and 3% diquafosol solution for dry eye patients: a randomized, blinded, multicenter clinical trial
- PMID: 31208393
- PMCID: PMC6580465
- DOI: 10.1186/s12886-019-1136-8
Comparison of 0.05% cyclosporine and 3% diquafosol solution for dry eye patients: a randomized, blinded, multicenter clinical trial
Retraction in
-
Retraction Note: Comparison of 0.05% cyclosporine and 3% diquafosol solution for dry eye patients: a randomized, blinded, multicenter clinical trial.BMC Ophthalmol. 2020 Jun 23;20(1):251. doi: 10.1186/s12886-020-01524-8. BMC Ophthalmol. 2020. PMID: 32576162 Free PMC article.
Abstract
Background: This study is aim to compare the clinical effectiveness between the two most prominent dry eye disease (DED)-specific eye drops, 0.05% cyclosporine (CN) and 3% diquafosol (DQ).
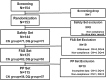
Methods: This is a multi-centered, randomized, masked, prospective clinical study. A total of 153 DED patients were randomly allocated to use CN twice per day or DQ six times daily. Cornea and conjunctival staining scores (NEI scale), tear break-up time (TBUT), Schirmer test scores, and ocular surface disease index (OSDI) score were measured at baseline, 4 and 12 weeks after treatment.
Results: At 12 weeks after treatment, NEI scaled scores were significantly reduced from the baseline by - 6.60 for CN and - 6.63 for DQ group (all P < 0.0001, P = 0.9739 between groups). TBUT and Schirmer values for CN were significantly improved from the baseline at 4 and 12 weeks (P = 0.0034, P < 0.0001 for TBUT, P = 0.0418, P = 0.0031 for Schirmer test). However, for DQ, TBUT showed significant improvement at 12 weeks only (P = 0.0281). Mean OSDI score differences from the baseline to 12 weeks were improved by - 13.03 ± 19.63 for CN and - 16.11 ± 20.87 for DQ, respectively (all P < 0.0001, P = 0.854 between groups). Regarding drug compliance, the mean instillation frequency of CN was less than that of DQ (P < 0.001). There were no statistically significant intergroup differences in safety evaluation.
Conclusions: The level of improvement regarding NEI, TBUT, and OSDI scores were not significantly different between the two treatment groups. However, with regards to the early improvement of TBUT and patient compliance, patients using CN improved faster and with greater adherence to drug usage than did those treated with DQ.
Trial registration: KCT0002180 , retrospectively registered on 23 December 2016.
Keywords: Cyclosporine; Diquafosol; Dry eye disease; Ocular surface disease index; Schirmer’s test; Tear break-up time.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

