New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence
- PMID: 31208414
- PMCID: PMC6580520
- DOI: 10.1186/s12933-019-0884-3
New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence
Abstract
Background: Saroglitazar, a novel dual peroxisome proliferator activated receptor (PPAR) agonist, in clinical trials, has shown an improvement in lipid and glycemic parameters through the PPAR-α and γ agonist actions, respectively. It was granted marketing authorization in India in 2013 for diabetic dyslipidemia. This review was conducted to summarize the effects of Saroglitazar in patients with diabetic dyslipidemia in real world clinical studies conducted after marketing authorization in India.
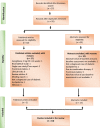
Methods: In this review, we selected real world clinical studies of Saroglitazar published as manuscripts and abstracts presented at scientific conferences. In all these studies, patients with diabetic dyslipidemia were treated with Saroglitazar 4 mg once daily for at least 12 weeks and different lipid and glycemic parameters were measured at the baseline and end of the study.
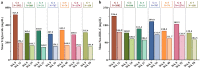
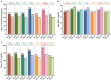
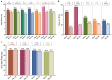
Results: In 18 selected studies (5 published manuscripts and 13 abstracts), a total of 5824 patients with diabetic dyslipidemia were prescribed Saroglitazar 4 mg for a duration ranging from 12 to 58 weeks. Across all the studies, mean age of patients ranged from 49.6 to 59.1 years and the proportion of female patients ranged from 22% to 42%. Across all the studies, there was a consistent mean reduction in triglyceride levels (~ 45% to 62%), total cholesterol levels (~ 17% to 26%), non-high-density lipoprotein cholesterol levels (~ 21% to 36%), low-density lipoprotein cholesterol levels (~ 11% to 27%), and glycosylated hemoglobin levels (~ 0.7% to 1.6%) with an increase in mean high-density lipoprotein cholesterol levels (up to 9%) from baseline to end of the study. Saroglitazar also improved alanine aminotransferase levels and fatty liver (evaluated by FibroScan™) in non-alcoholic fatty liver disease patients with diabetic dyslipidemia. Body weight remained unchanged and no significant adverse events (AEs) were reported in the studies.
Conclusion: Saroglitazar effectively improved lipid and glycemic parameters without significant AEs in patients with diabetic dyslipidemia in real-world clinical studies of up to 58 weeks duration.
Keywords: Alanine aminotransferase; Diabetic dyslipidemia; Dual PPAR agonist; Glycosylated hemoglobin; Non-alcoholic fatty liver disease; Saroglitazar; Triglyceride.
Conflict of interest statement
The authors declare that they have no competing interests with respect to the research, authorship, and/or publication of this review. DP is an employee of Zydus Discovery DMCC, Dubai, UAE. AJ is an employee of Zydus Healthcare Limited, Mumbai, India. MK, MS, KP, and KPP are employees of Zydus Research Centre, Cadila Healthcare Limited, Ahmedabad, India.
Figures
Similar articles
-
Effect of saroglitazar 2 mg and 4 mg on glycemic control, lipid profile and cardiovascular disease risk in patients with type 2 diabetes mellitus: a 56-week, randomized, double blind, phase 3 study (PRESS XII study).Cardiovasc Diabetol. 2020 Jun 19;19(1):93. doi: 10.1186/s12933-020-01073-w. Cardiovasc Diabetol. 2020. PMID: 32560724 Free PMC article. Clinical Trial.
-
Saroglitazar for the treatment of dyslipidemia in diabetic patients.Expert Opin Pharmacother. 2015 Mar;16(4):597-606. doi: 10.1517/14656566.2015.1009894. Expert Opin Pharmacother. 2015. PMID: 25674933 Review.
-
Structural Basis for Anti-non-alcoholic Fatty Liver Disease and Diabetic Dyslipidemia Drug Saroglitazar as a PPAR α/γ Dual Agonist.Biol Pharm Bull. 2021;44(9):1210-1219. doi: 10.1248/bpb.b21-00232. Biol Pharm Bull. 2021. PMID: 34471049
-
Observational study of effects of Saroglitazar on glycaemic and lipid parameters on Indian patients with type 2 diabetes.Sci Rep. 2015 Jan 9;5:7706. doi: 10.1038/srep07706. Sci Rep. 2015. PMID: 25573251 Free PMC article.
-
Effect of saroglitazar on glycaemic parameters: A systematic review and meta-analysis of randomized controlled trials.Diabetes Obes Metab. 2025 Sep;27(9):4627-4642. doi: 10.1111/dom.16506. Epub 2025 Jun 16. Diabetes Obes Metab. 2025. PMID: 40521795 Review.
Cited by
-
Pharmacological Therapeutics: Current Trends for Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD).J Clin Transl Hepatol. 2021 Dec 28;9(6):939-946. doi: 10.14218/JCTH.2021.00189. Epub 2021 Jul 28. J Clin Transl Hepatol. 2021. PMID: 34966657 Free PMC article. Review.
-
Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease.Biomolecules. 2023 Aug 18;13(8):1264. doi: 10.3390/biom13081264. Biomolecules. 2023. PMID: 37627329 Free PMC article. Review.
-
Effect of saroglitazar 2 mg and 4 mg on glycemic control, lipid profile and cardiovascular disease risk in patients with type 2 diabetes mellitus: a 56-week, randomized, double blind, phase 3 study (PRESS XII study).Cardiovasc Diabetol. 2020 Jun 19;19(1):93. doi: 10.1186/s12933-020-01073-w. Cardiovasc Diabetol. 2020. PMID: 32560724 Free PMC article. Clinical Trial.
-
Review article: the impact of liver-directed therapies on the atherogenic risk profile in non-alcoholic steatohepatitis.Aliment Pharmacol Ther. 2020 Aug;52(4):619-636. doi: 10.1111/apt.15935. Epub 2020 Jul 8. Aliment Pharmacol Ther. 2020. PMID: 32638417 Free PMC article. Review.
-
Saroglitazar suppresses KIM-1 and type IV collagen in high fat diet and low-dose streptozotocin-induced diabetic nephropathy in Wistar rats.Iran J Basic Med Sci. 2024;27(11):1447-1455. doi: 10.22038/ijbms.2024.78221.16908. Iran J Basic Med Sci. 2024. PMID: 39386240 Free PMC article.
References
-
- Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

