Bacterial alkylquinolone signaling contributes to structuring microbial communities in the ocean
- PMID: 31208456
- PMCID: PMC6580654
- DOI: 10.1186/s40168-019-0711-9
Bacterial alkylquinolone signaling contributes to structuring microbial communities in the ocean
Abstract
Background: Marine bacteria form complex relationships with eukaryotic hosts, from obligate symbioses to pathogenic interactions. These interactions can be tightly regulated by bioactive molecules, creating a complex system of chemical interactions through which these species chemically communicate thereby directly altering the host's physiology and community composition. Quorum sensing (QS) signals were first described in a marine bacterium four decades ago, and since then, we have come to discover that QS mediates processes within the marine carbon cycle, affects the health of coral reef ecosystems, and shapes microbial diversity and bacteria-eukaryotic host relationships. Yet, only recently have alkylquinolone signals been recognized for their role in cell-to-cell communication and the orchestration of virulence in biomedically relevant pathogens. The alkylquinolone, 2-heptyl-4-quinolone (HHQ), was recently found to arrest cell growth without inducing cell mortality in selected phytoplankton species at nanomolar concentrations, suggesting QS molecules like HHQ can influence algal physiology, playing pivotal roles in structuring larger ecological frameworks.
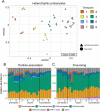
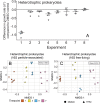
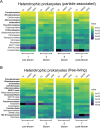
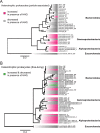
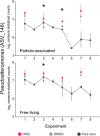
Results: To understand how natural communities of phytoplankton and bacteria respond to HHQ, field-based incubation experiments with ecologically relevant concentrations of HHQ were conducted over the course of a stimulated phytoplankton bloom. Bulk flow cytometry measurements indicated that, in general, exposure to HHQ caused nanoplankton and prokaryotic cell abundances to decrease. Amplicon sequencing revealed HHQ exposure altered the composition of particle-associated and free-living microbiota, favoring the relative expansion of both gamma- and alpha-proteobacteria, and a concurrent decrease in Bacteroidetes. Specifically, Pseudoalteromonas spp., known to produce HHQ, increased in relative abundance following HHQ exposure. A search of representative bacterial genomes from genera that increased in relative abundance when exposed to HHQ revealed that they all have the genetic potential to bind HHQ.
Conclusions: This work demonstrates HHQ has the capacity to influence microbial community organization, suggesting alkylquinolones have functions beyond bacterial communication and are pivotal in driving microbial community structure and phytoplankton growth. Knowledge of how bacterial signals alter marine communities will serve to deepen our understanding of the impact these chemical interactions have on a global scale.
Keywords: 2-heptyl-4-quinolone; Microbiome; Phytoplankton; Pseudoalteromonas; Quorum sensing.
Conflict of interest statement
The authors declare that they have no competing interests
Figures



References
-
- Danger M, Oumarou C, Benest D, Lacroix G. Bacteria can control stoichiometry and nutrient limitation of phytoplankton. Funct Ecol. 2007;21(2):202–210. doi: 10.1111/j.1365-2435.2006.01222.x. - DOI
-
- Fukami K, Nishimura S, Ogusa M, Asada M, Nishijima T. Continuous culture with deep seawater of a benthic food diatom Nitzschia sp. Hydrobiologia. 1997;358:245–249. doi: 10.1023/A:1003141104693. - DOI
-
- Azam F, Fenchel T, Field JG, Gray JS, Meyerreil LA, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 1983;10(3):257–263. doi: 10.3354/meps010257. - DOI
-
- Kumar Manoj, Ralph Peter., editors. Systems Biology of Marine Ecosystems. Cham: Springer International Publishing; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

