Recombinant peptide reverses cryo-capacitation in ram sperm and improves in vitro fertilization
- PMID: 31208850
- PMCID: PMC10699541
- DOI: 10.1016/j.anireprosci.2019.05.016
Recombinant peptide reverses cryo-capacitation in ram sperm and improves in vitro fertilization
Abstract
Semen cryopreservation is a very important technique for assisted reproduction; however, the cryopreservation process is harmful because it results in a reduction in sperm motility and viability, and leads to premature signals of capacitation, resulting in lesser than desirable fertility rates after artificial insemination. A fraction of seminal plasma, enriched in proteins that contain type II fibronectin domains (FNII) can reverse molecular indicators of cryo-capacitation. The beneficial effects of these proteins, however, depend on the relative abundance in seminal plasma. To create a safe additive for improving frozen sperm functionality, in the present study there was cloning and expression of a recombinant peptide containing four FNII domains (named TrxA-FNIIx4-His6) and evaluation of its effect after addition to frozen/thawed ram sperm. The cDNA for this protein was expressed in E. coli and after denaturation and re-naturalization of the protein, toxicity and binding capacity were assessed. By fluorescent labelling assessment, there was binding of the protein to the thawed sperm. At the two doses used (0.15 and 0.3 μM), TrxA-FNIIx4-His6 had the capacity to reverse the molecular indicators of cryo-capacitation as indicated by the reduction on phosphorylated substrates of PKA. Furthermore, the supplementation with this protein resulted in a normal capacitation process as evidenced by the increase in the in vitro fertilization rate when the greatest concentration of the protein was evaluated (73.25 ± 2.95; 40.13 ± 11.82 for 0.3 μM and control, respectively). There was no effect of protein supplementation on sperm objective motility compared to untreated sperm. In conclusion, the use of TrxA-FNIIx4-His6 is a promising biotechnological approach for cryopreserving ram sperm and maintaining sperm viability.
Keywords: Cryopreservation; Fibronectin (FNII) domains; Ram; Recombinant peptide; Seminal plasma.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interest
The authors have not declared any conflicts of interest.
Figures

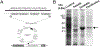
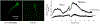



Similar articles
-
Boar seminal plasma inhibits cryo-capacitation of frozen-thawed ram sperm and improves fertility following intracervical insemination.Theriogenology. 2018 Jan 1;105:84-89. doi: 10.1016/j.theriogenology.2017.09.011. Epub 2017 Sep 18. Theriogenology. 2018. PMID: 28941408
-
Seminal plasma proteins interacting with sperm surface revert capacitation indicators in frozen-thawed ram sperm.Anim Reprod Sci. 2016 Oct;173:35-41. doi: 10.1016/j.anireprosci.2016.08.007. Epub 2016 Aug 16. Anim Reprod Sci. 2016. PMID: 27570190
-
Recombinant SPINK3 improves ram sperm quality and in vitro fertility after cryopreservation.Theriogenology. 2020 Mar 1;144:45-55. doi: 10.1016/j.theriogenology.2019.12.019. Epub 2019 Dec 27. Theriogenology. 2020. PMID: 31911322
-
Current status of sperm cryopreservation: why isn't it better?Theriogenology. 2002 Jan 1;57(1):327-44. doi: 10.1016/s0093-691x(01)00674-4. Theriogenology. 2002. PMID: 11775978 Review.
-
Cryopreservation of domestic animal sperm cells.Cell Tissue Bank. 2009 Feb;10(1):49-62. doi: 10.1007/s10561-008-9081-4. Epub 2008 Jun 12. Cell Tissue Bank. 2009. PMID: 18548333 Review.
Cited by
-
Palm Kernel Meal Protein Hydrolysates Enhance Post-Thawed Boar Sperm Quality.Animals (Basel). 2023 Sep 27;13(19):3040. doi: 10.3390/ani13193040. Animals (Basel). 2023. PMID: 37835646 Free PMC article.
-
Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality.Int J Mol Sci. 2020 Apr 16;21(8):2781. doi: 10.3390/ijms21082781. Int J Mol Sci. 2020. PMID: 32316334 Free PMC article. Review.
References
-
- Bailey J, Morrier A, Cormier N, 2003. Semen cryopreservation: successes and persistent problems in farm species. Can J. Anim. Sci 83, 393–401.
-
- Barrios B, Pérez-Pé R, Gallego M, Tato A, Osada J, Muiño-Blanco T, Cebrián Pérez J, 2000. Seminal plasma proteins revert the cold-shock damage on ram sperm membrane. Biol. Reprod 63, 1531–1537. - PubMed
-
- Barrios B, Fernández-Juan M, Muiño-Blanco T, Cebrián-Pérez J, 2005. Immunocytochemical localization and biochemical characterization of two seminal plasma proteins that protect ram sperm against cold shock. J. Androl 26, 539–549. - PubMed
-
- Bernardini A, Hozbor F, Sánchez E, Fornes M, Alberio R, Cesari A, 2011. Conserved ram seminal plasma proteins bind to the sperm membrane and repair cryopreservation damage. Theriogenology 76, 436–447. - PubMed
-
- Bradford M, 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72, 248–254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

