Correlates of HIV RNA concentrations in cerebrospinal fluid during antiretroviral therapy: a longitudinal cohort study
- PMID: 31208949
- PMCID: PMC12077812
- DOI: 10.1016/S2352-3018(19)30143-2
Correlates of HIV RNA concentrations in cerebrospinal fluid during antiretroviral therapy: a longitudinal cohort study
Abstract
Background: Few large projects have evaluated the factors that influence the HIV RNA concentrations (viral load) in cerebrospinal fluid (CSF) during antiretroviral therapy (ART) over time. We aimed to determine the correlates of HIV RNA in CSF in a large cohort.
Methods: We analysed longitudinal data from adults living with HIV in the US CHARTER cohort. Participants in the CHARTER study were recruited from six US academic medical centres-in Baltimore (MD), Galveston (TX), New York (NY), St Louis (MO), San Diego (C92A), and Seattle (WA). Participants in this study had been assessed at least three times between Sept 4, 2003, and Sept 14, 2010, and were taking ART and underwent venous and lumbar puncture with measurement of HIV RNA concentration at all assessments. The lower limit of quantification of the HIV RNA assays was 50 copies per mL. Data were analysed with longitudinal mixed effects logistic regression to identify correlates of HIV RNA concentration (as a binary [detectable or not] and as a continuous variable) in CSF over time. We tested demographic characteristics, plasma HIV RNA, nadir and current CD4 cell count in blood, current CD8 cell count in blood, estimated duration of HIV infection, AIDS diagnosis, duration of ART, adherence to ART, ART characteristics, and CSF characteristics as potential correlates.
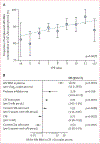
Findings: At the time of analysis, 2207 assessments from 401 participants met the criteria for inclusion in this study. Mean duration of observation was 33·7 months (range 12-84). HIV RNA concentrations in 710 (32·2%) plasma specimens and in 255 (11·6%) CSF specimens were greater than the lower limit of quantification. The best multivariate model of HIV RNA concentration in CSF greater than the lower limit of quantification over time included increased plasma HIV RNA concentration (odds ratio 18·0 per 1 log10 copy per mL, 95% CI 11·3 to 28·8; p<0·0001), increased CSF leucocyte count (2·01 per 5 cells per μL, 1·61 to 2·39; p<0·0001), decreased CD4 cell count (0·53 per 5 square-root cells per μL, 0·35 to 0·79; p=0·0025), decreased CNS penetration-effectiveness value (0·71 per unit, 0·56 to 0·92; p=0·0078), increased CD8 cell count (1·51 per 5 square-root cells, 1·11 to 2·06; p=0·0089), and protease inhibitor use (3·26, 1·04 to 10·23; p=0·039; model R2=0·22, p<0·0001). Analyses of continuous HIV RNA concentration in CSF that accounted for censoring below the lower limit of quantification had similar findings, although increased HIV RNA concentrations in CSF were also associated with black ethnicity (change in log10 HIV RNA concentration in CSF 0·205, 0·0367 to 0·3733; p=0·017), increased total protein in CSF (0·0025, -0·0002 to 0·0052; p=0·069), and the presence of addictive-drug metabolites in urine (0·103, -0·013 to 0·219; p=0·081).
Interpretation: The identified correlates of HIV RNA concentration in CSF during ART could strengthen clinical prediction of risk for failure to achieve or maintain HIV RNA suppression in CSF. Because most participants in this analysis were ART-experienced and were taking a three-drug regimen that did not include an integrase inhibitor, future research should focus on participants who are taking their first ART regimens or regimens that include integrase inhibitors or two drugs.
Funding: The work was supported by the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
DBC reports consultant fees from Biogen, Millennium/Takeda, Bristol-Myers Squibb, Genzyme (Sanofi), Pfizer, Amgen, Genentech/Roche, GlaxoSmithKline, Merck & Co/Serono, Inhibikase, Protagonist Therapeutics, and Shire Pharmaceuticals, and compensation as a Data Safety Monitoring Board member for Wave Pharmaceuticals. ACC reports compensation from Merck & Co as a Data Safety Monitoring Board member and by the International AIDS Society-USA for an educational lecture, and that research funding has been provided on her behalf to the University of Washington (WA, USA) by Bristol-Myers Squibb. SLL reports advisory board compensation from ViiV Healthcare and that research funding has been provided to the University of California, San Diego (CA, USA) on his behalf by Gilead Sciences and ViiV Healthcare. All other authors declare no competing interests.
Figures
Comment in
-
Markers of persistent HIV replication in the CNS.Lancet HIV. 2019 Jul;6(7):e416-e417. doi: 10.1016/S2352-3018(19)30182-1. Epub 2019 Jun 14. Lancet HIV. 2019. PMID: 31208950 No abstract available.
References
-
- Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis 1996; 174: 294–98. - PubMed
-
- Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol 1997; 42: 679–88. - PubMed
-
- McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol 1997; 42: 689–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

