MafB Is Important for Pancreatic β-Cell Maintenance under a MafA-Deficient Condition
- PMID: 31208980
- PMCID: PMC6692125
- DOI: 10.1128/MCB.00080-19
MafB Is Important for Pancreatic β-Cell Maintenance under a MafA-Deficient Condition
Abstract
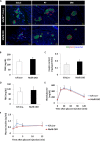
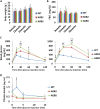
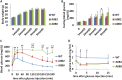
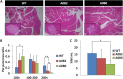
The pancreatic-islet-enriched transcription factors MafA and MafB have unique expression patterns in β cells in rodents. MafA is specifically expressed in β cells and is a key regulatory factor for maintaining adult β-cell function, whereas MafB plays an essential role in β-cell development during embryogenesis, and its expression in β cells gradually decreases and is restricted to α cells after birth in rodents. However, it was previously observed that MafB started to be reexpressed in insulin-positive (insulin+) β cells in MafA-deficient adult mice. To elucidate how MafB functions in the adult β cell under MafA-deficient conditions, we generated MafA and MafB double-knockout (A0B0) mice in which MafB was specifically deleted from β cells. As a result, the A0B0 mice became more vulnerable to diabetes under a high-fat diet (HFD) treatment, with impaired islet formation and a decreased number of insulin+ β cells because of increased β-cell apoptosis, indicating MafB can take part in the maintenance of adult β cells under certain pathological conditions.
Keywords: MafA; MafB; diabetes mellitus; pancreatic β cells.
Copyright © 2019 Xiafukaiti et al.
Figures


Similar articles
-
An Inducible Diabetes Mellitus Murine Model Based on MafB Conditional Knockout under MafA-Deficient Condition.Int J Mol Sci. 2020 Aug 5;21(16):5606. doi: 10.3390/ijms21165606. Int J Mol Sci. 2020. PMID: 32764399 Free PMC article.
-
Role of large MAF transcription factors in the mouse endocrine pancreas.Exp Anim. 2015;64(3):305-12. doi: 10.1538/expanim.15-0001. Epub 2015 Apr 27. Exp Anim. 2015. PMID: 25912440 Free PMC article.
-
Generation and characterization of MafA-Kusabira Orange mice.Endocr J. 2015;62(1):37-51. doi: 10.1507/endocrj.EJ14-0296. Epub 2014 Oct 1. Endocr J. 2015. PMID: 25273397
-
MafA and MafB activity in pancreatic β cells.Trends Endocrinol Metab. 2011 Sep;22(9):364-73. doi: 10.1016/j.tem.2011.05.003. Epub 2011 Jun 28. Trends Endocrinol Metab. 2011. PMID: 21719305 Free PMC article. Review.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
-
Developmentally dynamic changes in DNA methylation in the human pancreas.BMC Genomics. 2024 Jun 3;25(1):553. doi: 10.1186/s12864-024-10450-8. BMC Genomics. 2024. PMID: 38831310 Free PMC article.
-
Ginger extract promotes pancreatic islets regeneration in streptozotocin-induced diabetic rats.Biosci Rep. 2025 Mar 13;45(3):BSR20241510. doi: 10.1042/BSR20241510. Biosci Rep. 2025. PMID: 40014427 Free PMC article.
-
Mapping cells through time and space with moscot.Nature. 2025 Feb;638(8052):1065-1075. doi: 10.1038/s41586-024-08453-2. Epub 2025 Jan 22. Nature. 2025. PMID: 39843746 Free PMC article.
-
Pancreatic Islets and Gestalt Principles.Diabetes. 2020 Sep;69(9):1864-1874. doi: 10.2337/db20-0304. Epub 2020 Jul 15. Diabetes. 2020. PMID: 32669392 Free PMC article.
-
Epigallocatechin-3-gallate alleviates type 2 diabetes mellitus via β-cell function improvement and insulin resistance reduction.Iran J Basic Med Sci. 2022 Apr;25(4):483-488. doi: 10.22038/IJBMS.2022.58591.13016. Iran J Basic Med Sci. 2022. PMID: 35656076 Free PMC article.
References
-
- Alberti KG, Zimmet PZ. 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part1. Diagnosis and classification of diabetes mellitus; provisional report of a WHO consultation. Diabet Med 15:539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
