Chemotropic signaling by BMP7 requires selective interaction at a key residue in ActRIIA
- PMID: 31208997
- PMCID: PMC6679395
- DOI: 10.1242/bio.042283
Chemotropic signaling by BMP7 requires selective interaction at a key residue in ActRIIA
Abstract
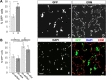
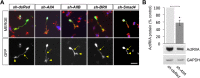
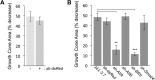
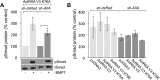
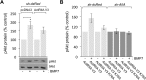
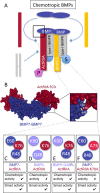
BMP7 evokes acute chemotropic PI3K-dependent responses, such as growth cone collapse and monocyte chemotaxis, as well as classical Smad-dependent gene transcription. That these divergent responses can be activated in the same cell raises the question of how the BMP-dependent signaling apparatus is manipulated to produce chemotropic and transcriptional signals. RNA interference and site-directed mutagenesis were used to explore functional and structural BMP receptor requirements for BMP7-evoked chemotropic activity. We show that specific type II BMP receptor subunits, ActRIIA and BMPR2, are required for BMP7-induced growth cone collapse in developing spinal neurons and for chemotaxis of monocytes. Reintroduction of wild-type ActRIIA into monocytic cells lacking endogenous ActRIIA restores BMP7-evoked chemotaxis, whereas expression of an ActRIIA K76A receptor variant fails to rescue. BMP7-evoked Smad-dependent signaling is unaffected by either ActRIIA knockdown or expression of the ActRIIA K76A variant. In contrast, BMP7-evoked PI3K-dependent signaling is significantly disturbed in the presence of ActRIIA K76A. These results support a model for selective engagement of chemotropic BMPs with type II BMP receptors, through specific residues, that results in strict regulation of PI3K-dependent signal transduction.This article has an associated First Person interview with the first author of the paper.
Keywords: ActRIIA; BMP7; Chemotaxis; Growth cone collapse; PI3K.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
ActRIIA and BMPRII Type II BMP receptor subunits selectively required for Smad4-independent BMP7-evoked chemotaxis.PLoS One. 2009 Dec 8;4(12):e8198. doi: 10.1371/journal.pone.0008198. PLoS One. 2009. PMID: 20011660 Free PMC article.
-
Structural distinctions in BMPs underlie divergent signaling in spinal neurons.Neural Dev. 2012 May 4;7:16. doi: 10.1186/1749-8104-7-16. Neural Dev. 2012. PMID: 22559862 Free PMC article.
-
Inductive specification and axonal orientation of spinal neurons mediated by divergent bone morphogenetic protein signaling pathways.Neural Dev. 2011 Nov 15;6:36. doi: 10.1186/1749-8104-6-36. Neural Dev. 2011. PMID: 22085733 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer.Endocr J. 2008 Mar;55(1):11-21. doi: 10.1507/endocrj.kr-110. Epub 2007 Sep 14. Endocr J. 2008. PMID: 17878607 Review.
Cited by
-
Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: therapeutic potential for endogenous pulp regeneration.Int J Oral Sci. 2022 Jul 20;14(1):38. doi: 10.1038/s41368-022-00188-y. Int J Oral Sci. 2022. PMID: 35858911 Free PMC article.
-
DNA methylation patterns in umbilical cord blood from infants of methadone maintained opioid dependent mothers.Sci Rep. 2024 Jul 27;14(1):17298. doi: 10.1038/s41598-024-66899-w. Sci Rep. 2024. PMID: 39068260 Free PMC article.
-
A Pharmacoinformatics Analysis of Artemisinin Targets and de novo Design of Hits for Treating Ulcerative Colitis.Front Pharmacol. 2022 Mar 18;13:843043. doi: 10.3389/fphar.2022.843043. eCollection 2022. Front Pharmacol. 2022. PMID: 35370688 Free PMC article.
-
Discovery of a novel class of benzimidazoles as highly effective agonists of bone morphogenetic protein (BMP) receptor signaling.Sci Rep. 2022 Jul 15;12(1):12146. doi: 10.1038/s41598-022-16394-x. Sci Rep. 2022. PMID: 35840622 Free PMC article.
-
Assessment of BMP responses in an in vitro model of acute ethanol toxicity.MethodsX. 2022 Feb 6;9:101631. doi: 10.1016/j.mex.2022.101631. eCollection 2022. MethodsX. 2022. PMID: 35242615 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

