Pharmacodynamics of Isavuconazole in a Rabbit Model of Cryptococcal Meningoencephalitis
- PMID: 31209006
- PMCID: PMC6709487
- DOI: 10.1128/AAC.00546-19
Pharmacodynamics of Isavuconazole in a Rabbit Model of Cryptococcal Meningoencephalitis
Abstract
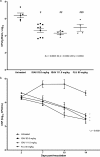
Cryptococcus spp., important fungal pathogens, are the leading cause of fungus-related mortality in human immunodeficiency virus-infected patients, and new therapeutic options are desperately needed. Isavuconazonium sulfate, a newer triazole antifungal agent, was studied to characterize the exposure-response relationship in a rabbit model of cryptococcal meningoencephalitis. Rabbits treated with isavuconazonium sulfate were compared with those treated with fluconazole and untreated controls. The fungal burden in the cerebrospinal fluid was measured serially over time, while the yeast concentrations in the brain and the eye (aqueous humor) were determined at the end of therapy. The exposure impact of isavuconazonium sulfate dosing in the rabbit was linked using mathematical modeling. Similar significant reductions in the fungal burden in the brain and cerebrospinal fluid in rabbits treated with isavuconazonium sulfate and fluconazole compared with that in the untreated controls were observed. No dose-dependent response was demonstrated with isavuconazonium sulfate treatment in this study. The treatment of cryptococcal meningoencephalitis with isavuconazonium sulfate was similar to that with fluconazole. Dose-dependent reductions in yeast over time were not demonstrated, which limited our ability to estimate the pharmacodynamic target. Further nonclinical and clinical studies are needed in order to characterize the extent of the exposure-response relationship in cryptococcal meningoencephalitis. However, this study suggests that isavuconazonium sulfate, like fluconazole, could be beneficial in the setting of consolidation and maintenance therapy, rather than induction monotherapy, in high-burden cryptococcal meningoencephalitis.
Keywords: Cryptococcus neoformans; cryptococcosis; fluconazole; isavuconazole; isavuconazonium sulfate; meningoencephalitis.
Copyright © 2019 Kovanda et al.
Figures
References
-
- World Health Organization. 2018. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO Guidelines Approved by the Guidelines Review Committee, World Health Organization, Geneva, Switzerland: https://www.ncbi.nlm.nih.gov/books/NBK531449/. Accessed 25 June 2019. - PubMed
-
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi:10.1093/cid/cit794. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

