Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints
- PMID: 31209017
- PMCID: PMC6613080
- DOI: 10.1073/pnas.1902035116
Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints
Abstract
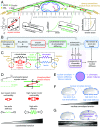
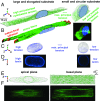
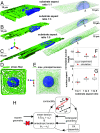
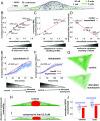
Cells sense mechanical signals from their microenvironment and transduce them to the nucleus to regulate gene expression programs. To elucidate the physical mechanisms involved in this regulation, we developed an active 3D chemomechanical model to describe the three-way feedback between the adhesions, the cytoskeleton, and the nucleus. The model shows local tensile stresses generated at the interface of the cell and the extracellular matrix regulate the properties of the nucleus, including nuclear morphology, levels of lamin A,C, and histone deacetylation, as these tensile stresses 1) are transmitted to the nucleus through cytoskeletal physical links and 2) trigger an actomyosin-dependent shuttling of epigenetic factors. We then show how cell geometric constraints affect the local tensile stresses and subsequently the three-way feedback and induce cytoskeleton-mediated alterations in the properties of the nucleus such as nuclear lamina softening, chromatin stiffening, nuclear lamina invaginations, increase in nuclear height, and shrinkage of nuclear volume. We predict a phase diagram that describes how the disruption of cytoskeletal components impacts the feedback and subsequently induce contractility-dependent alterations in the properties of the nucleus. Our simulations show that these changes in contractility levels can be also used as predictors of nucleocytoplasmic shuttling of transcription factors and the level of chromatin condensation. The predictions are experimentally validated by studying the properties of nuclei of fibroblasts on micropatterned substrates with different shapes and areas.
Keywords: cell geometry; cytoskeletal mechanics; mechanotransduction; nuclear mechanics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Discher D. E., Janmey P., Wang Y. L., Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

