Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis
- PMID: 31209025
- PMCID: PMC6613144
- DOI: 10.1073/pnas.1904170116
Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis
Abstract
Lyme disease is a multisystem disorder caused by the spirochete Borrelia burgdorferi A common late-stage complication of this disease is oligoarticular arthritis, often involving the knee. In ∼10% of cases, arthritis persists after appropriate antibiotic treatment, leading to a proliferative synovitis typical of chronic inflammatory arthritides. Here, we provide evidence that peptidoglycan (PG), a major component of the B. burgdorferi cell envelope, may contribute to the development and persistence of Lyme arthritis (LA). We show that B. burgdorferi has a chemically atypical PG (PGBb) that is not recycled during cell-wall turnover. Instead, this pathogen sheds PGBb fragments into its environment during growth. Patients with LA mount a specific immunoglobulin G response against PGBb, which is significantly higher in the synovial fluid than in the serum of the same patient. We also detect PGBb in 94% of synovial fluid samples (32 of 34) from patients with LA, many of whom had undergone oral and intravenous antibiotic treatment. These same synovial fluid samples contain proinflammatory cytokines, similar to those produced by human peripheral blood mononuclear cells stimulated with PGBb In addition, systemic administration of PGBb in BALB/c mice elicits acute arthritis. Altogether, our study identifies PGBb as a likely contributor to inflammatory responses in LA. Persistence of this antigen in the joint may contribute to synovitis after antibiotics eradicate the pathogen. Furthermore, our finding that B. burgdorferi sheds immunogenic PGBb fragments during growth suggests a potential role for PGBb in the immunopathogenesis of other Lyme disease manifestations.
Keywords: Borrelia burgdorferi; Lyme disease; arthritis; inflammation; peptidoglycan.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
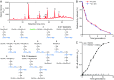


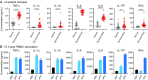

Similar articles
-
Synthesis of a Borrelia burgdorferi-Derived Muropeptide Standard Fragment Library.Molecules. 2024 Jul 12;29(14):3297. doi: 10.3390/molecules29143297. Molecules. 2024. PMID: 39064876 Free PMC article.
-
Bacterial and host enzymes modulate the pro-inflammatory response elicited by the peptidoglycan of Lyme disease agent Borrelia burgdorferi.PLoS Pathog. 2025 Jul 7;21(7):e1013324. doi: 10.1371/journal.ppat.1013324. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40623106 Free PMC article.
-
Borrelia peptidoglycan interacting Protein (BpiP) contributes to the fitness of Borrelia burgdorferi against host-derived factors and influences virulence in mouse models of Lyme disease.PLoS Pathog. 2021 Apr 21;17(4):e1009535. doi: 10.1371/journal.ppat.1009535. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33882111 Free PMC article.
-
The role of host immune cells and Borrelia burgdorferi antigens in the etiology of Lyme disease.Eur Cytokine Netw. 2017 Jun 1;28(2):70-84. doi: 10.1684/ecn.2017.0396. Eur Cytokine Netw. 2017. PMID: 28840838 Review. English.
-
Lyme arthritis and post-Lyme disease syndrome.Curr Opin Rheumatol. 2002 Jul;14(4):383-7. doi: 10.1097/00002281-200207000-00008. Curr Opin Rheumatol. 2002. PMID: 12118171 Review.
Cited by
-
Heightened Proinflammatory Glycosylation of Borrelia burgdorferi IgG Antibodies in Synovial Fluid in Patients With Antibiotic-Refractory Lyme Arthritis.Arthritis Rheumatol. 2023 Jul;75(7):1263-1274. doi: 10.1002/art.42465. Epub 2023 May 11. Arthritis Rheumatol. 2023. PMID: 36716113 Free PMC article.
-
The Cdkn2a gene product p19 alternative reading frame (p19ARF) is a critical regulator of IFNβ-mediated Lyme arthritis.PLoS Pathog. 2022 Mar 24;18(3):e1010365. doi: 10.1371/journal.ppat.1010365. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35324997 Free PMC article.
-
Lyme disease: implications for general practice.Br J Gen Pract. 2020 Feb 27;70(692):106-107. doi: 10.3399/bjgp20X708341. Print 2020 Mar. Br J Gen Pract. 2020. PMID: 32107217 Free PMC article. No abstract available.
-
A host lipase prevents lipopolysaccharide-induced foam cell formation.iScience. 2021 Aug 19;24(9):103004. doi: 10.1016/j.isci.2021.103004. eCollection 2021 Sep 24. iScience. 2021. PMID: 34522852 Free PMC article.
-
Lyme arthritis: linking infection, inflammation and autoimmunity.Nat Rev Rheumatol. 2021 Aug;17(8):449-461. doi: 10.1038/s41584-021-00648-5. Epub 2021 Jul 5. Nat Rev Rheumatol. 2021. PMID: 34226730 Free PMC article. Review.
References
-
- Mead P. S., Epidemiology of Lyme disease. Infect. Dis. Clin. North Am. 29, 187–210 (2015). - PubMed
-
- Stanek G., Strle F., Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 42, 233–258 (2018). - PubMed
-
- Koedel U., Fingerle V., Pfister H. W., Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 11, 446–456 (2015). - PubMed
-
- Robinson M. L., Kobayashi T., Higgins Y., Calkins H., Melia M. T., Lyme carditis. Infect. Dis. Clin. North Am. 29, 255–268 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

