Altered Stool Microbiota of Infants with Cystic Fibrosis Shows a Reduction in Genera Associated with Immune Programming from Birth
- PMID: 31209076
- PMCID: PMC6657602
- DOI: 10.1128/JB.00274-19
Altered Stool Microbiota of Infants with Cystic Fibrosis Shows a Reduction in Genera Associated with Immune Programming from Birth
Abstract
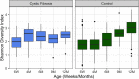
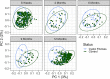
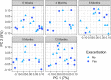
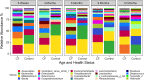
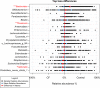
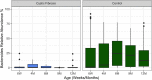
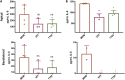
Previous work from our group indicated an association between the gastrointestinal microbiota of infants with cystic fibrosis (CF) and airway disease in this population. Here we report that stool microbiota of infants with CF demonstrates an altered but largely unchanging within-individual bacterial diversity (alpha diversity) over the first year of life, in contrast to the infants without CF (control cohort), which showed the expected increase in alpha diversity over the first year. The beta diversity, or between-sample diversity, of these two cohorts was significantly different over the first year of life and was statistically significantly associated with airway exacerbations, confirming our earlier findings. Compared with control infants, infants with CF had reduced levels of Bacteroides, a bacterial genus associated with immune modulation, as early as 6 weeks of life, and this significant reduction of Bacteroides spp. in the cohort with CF persisted over the entire first year of life. Only two other genera were significantly different across the first year of life: Roseburia was significantly reduced and Veillonella was significantly increased. Other genera showed differences between the two cohorts but only at selected time points. In vitro studies demonstrated that exposure of the apical face of polarized intestinal cell lines to Bacteroides species supernatants significantly reduced production of interleukin 8 (IL-8), suggesting a mechanism whereby changes in the intestinal microbiota could impact inflammation in CF. This work further establishes an association between gastrointestinal microbiota, inflammation, and airway disease in infants with CF and presents a potential opportunity for therapeutic interventions beginning in early life.IMPORTANCE There is growing evidence for a link between gastrointestinal bacterial communities and airway disease progression in CF. We demonstrate that infants with CF ≤1 year of age show a distinct stool microbiota versus that of control infants of a comparable age. We detected associations between the gut microbiome and airway exacerbation events in the cohort of infants with CF, and in vitro studies provided one possible mechanism for this observation. These data clarify that current therapeutics do not establish in infants with CF a gastrointestinal microbiota like that in healthy infants, and we suggest that interventions that direct the gastrointestinal microbiota closer to a healthy state may provide systemic benefits to these patients during a critical window of immune programming that might have implications for lifelong health.
Keywords: cystic fibrosis; cytokine; exacerbation; infant; intestine; microbiota; stool.
Copyright © 2019 American Society for Microbiology.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

