Study protocol of a multicentre cohort pilot study implementing an expanded preconception carrier-screening programme in metropolitan and regional Western Australia
- PMID: 31209093
- PMCID: PMC6589024
- DOI: 10.1136/bmjopen-2018-028209
Study protocol of a multicentre cohort pilot study implementing an expanded preconception carrier-screening programme in metropolitan and regional Western Australia
Abstract
Introduction: Preconception carrier screening (PCS) identifies couples at risk of having children with recessive genetic conditions. New technologies have enabled affordable sequencing for multiple disorders simultaneously, including identifying carrier status for many recessive diseases. The aim of the study was to identify the most effective way of delivering PCS in Western Australia (WA) through the public health system.
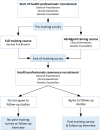
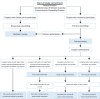
Methods and analysis: This is a multicentre cohort pilot study of 250 couples who have used PCS, conducted at three sites: (1) Genetic Services of Western Australia, (2) a private genetic counselling practice in Perth and (3) participating general practice group practices in the Busselton region of WA. The primary outcome of the pilot study was to evaluate the feasibility of implementing the comprehensive PCS programme in the WA healthcare system. Secondary outcome measures included evaluation of the psychosocial impact of couples, such as reproductive autonomy; identification of areas within the health system that had difficulties in implementing the programme and evaluation of tools developed during the study.
Ethics and dissemination: Approval was provided by the Women and Newborn Health Service Human Research Ethics Committee (HREC) at King Edward Memorial Hospital for Women (RGS0000000946) and the University of Western Australia (UWA) HREC (RA/4/20/4258). Participants may choose to withdraw at any time. Withdrawal will in no way affect participating couples' medical care. Study couples will be redirected to another participating health professional for consultation or counselling in the event of a health professional withdrawing. All evaluation data will be deidentified and stored in a password-protected database in UWA. In addition, all hard copy data collected will be kept in a locked cabinet within a secure building. All electronic data will be stored in a password-protected, backed-up location in the UWA Institutional Research Data Store. All evaluative results will be published as separate manuscripts, and selected results will be presented at conferences.
Keywords: attitudes; genetic carrier screening; knowledge; pilot study protocol; reproductive medicine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Measuring the impact of genetic knowledge on intentions and attitudes of the community towards expanded preconception carrier screening.J Med Genet. 2018 Nov;55(11):744-752. doi: 10.1136/jmedgenet-2018-105362. Epub 2018 Aug 1. J Med Genet. 2018. PMID: 30068663
-
Attitudes of the general population towards preconception expanded carrier screening for autosomal recessive disorders including inborn errors of metabolism.Mol Genet Metab. 2019 Jan;126(1):14-22. doi: 10.1016/j.ymgme.2018.12.004. Epub 2018 Dec 10. Mol Genet Metab. 2019. PMID: 30563741
-
Preconception carrier screening in couples seeking IVF: exploring the patient perspective.Reprod Biomed Online. 2025 Jan;50(1):104452. doi: 10.1016/j.rbmo.2024.104452. Epub 2024 Sep 21. Reprod Biomed Online. 2025. PMID: 39647447
-
Interest in expanded carrier screening among individuals and couples in the general population: systematic review of the literature.Hum Reprod Update. 2020 Apr 15;26(3):335-355. doi: 10.1093/humupd/dmaa001. Hum Reprod Update. 2020. PMID: 32099997
-
Expanded carrier screening: A current perspective.Eur J Obstet Gynecol Reprod Biol. 2018 Nov;230:41-54. doi: 10.1016/j.ejogrb.2018.09.014. Epub 2018 Sep 13. Eur J Obstet Gynecol Reprod Biol. 2018. PMID: 30240948 Review.
Cited by
-
Societal implications of expanded universal carrier screening: a scoping review.Eur J Hum Genet. 2023 Jan;31(1):55-72. doi: 10.1038/s41431-022-01178-8. Epub 2022 Sep 12. Eur J Hum Genet. 2023. PMID: 36097155 Free PMC article.
-
Attitudes of professional stakeholders towards implementation of reproductive genetic carrier screening: a systematic review.Eur J Hum Genet. 2023 Apr;31(4):395-408. doi: 10.1038/s41431-022-01274-9. Epub 2023 Jan 12. Eur J Hum Genet. 2023. PMID: 36631542 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
