The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout-Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1β Release
- PMID: 31209100
- PMCID: PMC6650356
- DOI: 10.4049/jimmunol.1900228
The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout-Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1β Release
Abstract
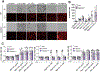
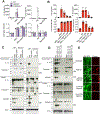
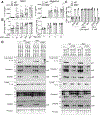
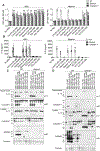
The pyroptotic cell death effector gasdermin D (GSDMD) is required for murine models of hereditary inflammasome-driven, IL-1β-dependent, autoinflammatory disease, making it an attractive therapeutic target. However, the importance of GSDMD for more common conditions mediated by pathological IL-1β activation, such as gout, remain unclear. In this study, we address whether GSDMD and the recently described GSDMD inhibitor necrosulfonamide (NSA) contribute to monosodium urate (MSU) crystal-induced cell death, IL-1β release, and autoinflammation. We demonstrate that MSU crystals, the etiological agent of gout, rapidly activate GSDMD in murine macrophages. Despite this, the genetic deletion of GSDMD or the other lytic effector implicated in MSU crystal killing, mixed lineage kinase domain-like (MLKL), did not prevent MSU crystal-induced cell death. Consequently, GSDMD or MLKL loss did not hinder MSU crystal-mediated release of bioactive IL-1β. Consistent with in vitro findings, IL-1β induction and autoinflammation in MSU crystal-induced peritonitis was not reduced in GSDMD-deficient mice. Moreover, we show that the reported GSDMD inhibitor, NSA, blocks inflammasome priming and caspase-1 activation, thereby preventing pyroptosis independent of GSDMD targeting. The inhibition of cathepsins, widely implicated in particle-induced macrophage killing, also failed to prevent MSU crystal-mediated cell death. These findings 1) demonstrate that not all IL-1β-driven autoinflammatory conditions will benefit from the therapeutic targeting of GSDMD, 2) document a unique mechanism of MSU crystal-induced macrophage cell death not rescued by pan-cathepsin inhibition, and 3) show that NSA inhibits inflammasomes upstream of GSDMD to prevent pyroptotic cell death and IL-1β release.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures



References
-
- Kuo CF, Grainge MJ, Zhang W, and Doherty M. 2015. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 11: 649–662. - PubMed
-
- Edwards NL 2011. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr Rheumatol Rep 13: 154–159. - PubMed
-
- Mulay SR, and Anders HJ. 2016. Crystallopathies. N Engl J Med 374: 2465–2476. - PubMed
-
- Martinon F, Pétrilli V, Mayor A, Tardivel A, and Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241. - PubMed
-
- So AK, and Martinon F. 2017. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol 13: 639–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

