Acetyl-CoA carboxylase 1-dependent lipogenesis promotes autophagy downstream of AMPK
- PMID: 31209110
- PMCID: PMC6690696
- DOI: 10.1074/jbc.RA118.007020
Acetyl-CoA carboxylase 1-dependent lipogenesis promotes autophagy downstream of AMPK
Abstract
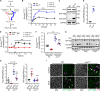
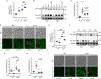
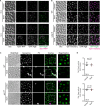
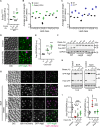
Autophagy, a membrane-dependent catabolic process, ensures survival of aging cells and depends on the cellular energetic status. Acetyl-CoA carboxylase 1 (Acc1) connects central energy metabolism to lipid biosynthesis and is rate-limiting for the de novo synthesis of lipids. However, it is unclear how de novo lipogenesis and its metabolic consequences affect autophagic activity. Here, we show that in aging yeast, autophagy levels highly depend on the activity of Acc1. Constitutively active Acc1 (acc1S/A ) or a deletion of the Acc1 negative regulator, Snf1 (yeast AMPK), shows elevated autophagy levels, which can be reversed by the Acc1 inhibitor soraphen A. Vice versa, pharmacological inhibition of Acc1 drastically reduces cell survival and results in the accumulation of Atg8-positive structures at the vacuolar membrane, suggesting late defects in the autophagic cascade. As expected, acc1S/A cells exhibit a reduction in acetate/acetyl-CoA availability along with elevated cellular lipid content. However, concomitant administration of acetate fails to fully revert the increase in autophagy exerted by acc1S/A Instead, administration of oleate, while mimicking constitutively active Acc1 in WT cells, alleviates the vacuolar fusion defects induced by Acc1 inhibition. Our results argue for a largely lipid-dependent process of autophagy regulation downstream of Acc1. We present a versatile genetic model to investigate the complex relationship between acetate metabolism, lipid homeostasis, and autophagy and propose Acc1-dependent lipogenesis as a fundamental metabolic path downstream of Snf1 to maintain autophagy and survival during cellular aging.
Keywords: AMPK; Acc1; Snf1; acetate; acetyl coenzyme A (acetyl-CoA); acetyl-CoA carboxylase 1; aging; autophagy; lipid metabolism; lipogenesis; oleate; yeast.
© 2019 Gross et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Inhibition of acetyl coenzyme A carboxylase activity restores expression of the INO1 gene in a snf1 mutant strain of Saccharomyces cerevisiae.Mol Cell Biol. 2001 Sep;21(17):5710-22. doi: 10.1128/MCB.21.17.5710-5722.2001. Mol Cell Biol. 2001. PMID: 11486011 Free PMC article.
-
Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1.mBio. 2014 May 6;5(3):e01130-14. doi: 10.1128/mBio.01130-14. mBio. 2014. PMID: 24803522 Free PMC article.
-
Fine-tuning acetyl-CoA carboxylase 1 activity through localization: functional genomics reveals a role for the lysine acetyltransferase NuA4 and sphingolipid metabolism in regulating Acc1 activity and localization.Genetics. 2022 Jul 30;221(4):iyac086. doi: 10.1093/genetics/iyac086. Genetics. 2022. PMID: 35608294 Free PMC article.
-
Platelet lipidomics and de novo lipogenesis: impact on health and disease.Curr Opin Hematol. 2024 Sep 1;31(5):217-223. doi: 10.1097/MOH.0000000000000820. Epub 2024 May 7. Curr Opin Hematol. 2024. PMID: 38727017 Free PMC article. Review.
-
Acetyl-CoA carboxylase-a as a novel target for cancer therapy.Front Biosci (Schol Ed). 2010 Jan 1;2(2):515-26. doi: 10.2741/s82. Front Biosci (Schol Ed). 2010. PMID: 20036965 Review.
Cited by
-
Lipid and glucose metabolism in senescence.Front Nutr. 2023 Aug 23;10:1157352. doi: 10.3389/fnut.2023.1157352. eCollection 2023. Front Nutr. 2023. PMID: 37680899 Free PMC article. Review.
-
Apelin-13 Protects Dopaminergic Neurons against Rotenone-Induced Neurotoxicity through the AMPK/mTOR/ULK-1 Mediated Autophagy Activation.Int J Mol Sci. 2020 Nov 8;21(21):8376. doi: 10.3390/ijms21218376. Int J Mol Sci. 2020. PMID: 33171641 Free PMC article.
-
Phospholipid Scramblase Activity of VDAC Dimers: New Implications for Cell Death, Autophagy and Ageing.Biomolecules. 2024 Sep 26;14(10):1218. doi: 10.3390/biom14101218. Biomolecules. 2024. PMID: 39456151 Free PMC article. Review.
-
The sensor histidine kinase (SLN1) and acetyl-CoA carboxylase (ACC1) coordinately regulate the response of Neurospora crassa to the springtail Sinella curviseta (Collembola: Entomobryidae) attack.Appl Environ Microbiol. 2023 Nov 29;89(11):e0101823. doi: 10.1128/aem.01018-23. Epub 2023 Oct 19. Appl Environ Microbiol. 2023. PMID: 37855634 Free PMC article.
-
In S. cerevisiae hydroxycitric acid antagonizes chronological aging and apoptosis regardless of citrate lyase.Apoptosis. 2020 Oct;25(9-10):686-696. doi: 10.1007/s10495-020-01625-1. Apoptosis. 2020. PMID: 32666259 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

