Anterior Cingulate Cortex and Ventral Hippocampal Inputs to the Basolateral Amygdala Selectively Control Generalized Fear
- PMID: 31209172
- PMCID: PMC6697404
- DOI: 10.1523/JNEUROSCI.0810-19.2019
Anterior Cingulate Cortex and Ventral Hippocampal Inputs to the Basolateral Amygdala Selectively Control Generalized Fear
Abstract
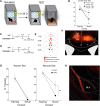
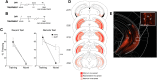
A common symptom of anxiety disorders is the overgeneralization of fear across a broad range of contextual cues. We previously found that the ACC and ventral hippocampus (vHPC) regulate generalized fear. Here, we investigate the functional projections from the ACC and vHPC to the amygdala and their role in governing generalized fear in a preclinical rodent model. A chemogenetic approach (designer receptor exclusively activated by designer drugs) was used to inhibit glutamatergic projections from the ACC or vHPC that terminate within the BLA at recent (1 d) or remote (28 d) time points after contextually fear conditioning male mice. Inactivating ACC or vHPC projections to the BLA significantly reduced generalized fear to a novel, nonthreatening context but had no effect on fear to the training context. Further, our data indicate that the ACC-BLA circuit supports generalization in a time-independent manner. We also identified, for the first time, a strictly time-dependent role of the vHPC-BLA circuit in supporting remote generalized contextual fear. Dysfunctional signaling to the amygdala from the ACC or the HPC could underlie overgeneralized fear responses that are associated with anxiety disorders. Our findings demonstrate that the ACC and vHPC regulate fear expressed in novel, nonthreatening environments via projections to the BLA but do so as a result of training intensity or time, respectively.SIGNIFICANCE STATEMENT Anxiety disorders are characterized by a common symptom that promotes overgeneralization of fear in nonthreatening environments. Dysregulation of the amygdala, ACC, or hippocampus (HPC) has been hypothesized to contribute to increased fear associated with anxiety disorders. Our findings show that the ACC and HPC projections to the BLA regulate generalized fear in nonthreatening, environments. However, descending ACC projections control fear generalization independent of time, whereas HPC projections play a strictly time-dependent role in regulating generalized fear. Thus, dysfunctional ACC/HPC signaling to the BLA may be a predominant underlying mechanism of nonspecific fear associated with anxiety disorders. Our data have important implications for predictions made by theories about aging memories and interactions between the HPC and cortical regions.
Keywords: ACC; DREADD; amygdala; anxiety disorders; context generalization; hippocampus.
Copyright © 2019 the authors.
Figures




References
-
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lépine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, et al. (2004) Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. Acta Psychiatr Scand Suppl 109:21–27. 10.1111/j.1600-0047.2004.00327.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
